Describe the electrolytic cell shown in the schematic at points 1, 2, 3, and 4. Include relevant half reactions for points 2 and 4.
Understand the Problem
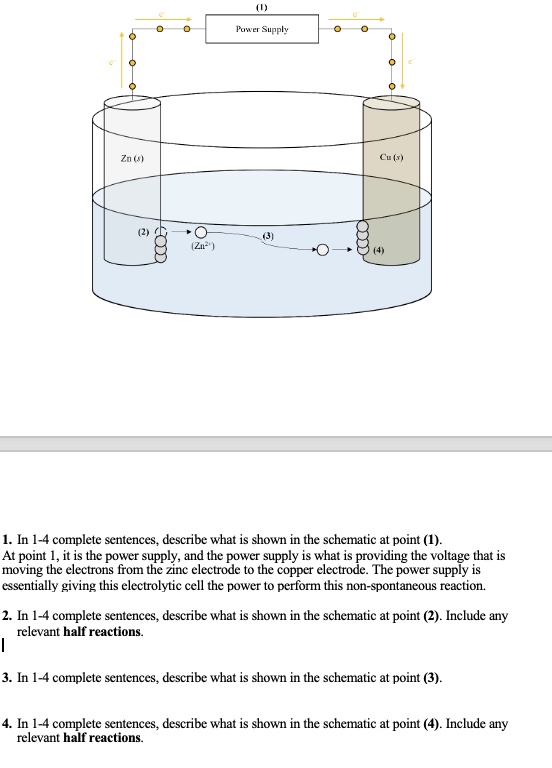
The image shows an electrolytic cell with zinc and copper electrodes. The questions ask for descriptions of what is happening at different points in the cell, specifically focusing on the power supply (1), the zinc electrode (2), the ion transport (3), and the copper electrode (4). For points (2) and (4), half-reactions need to be included.
Answer
* **Point 1:** Power supply. * **Point 2:** Zinc electrode, Zn(s) → Zn²⁺(aq) + 2e⁻. * **Point 3:** Zinc ions moving. * **Point 4:** Copper electrode, Cu²⁺(aq) + 2e⁻ → Cu(s).
Here are the descriptions for the requested points:
- Point 1: It's a power supply providing voltage, moving electrons from the zinc electrode to the copper electrode, enabling the non-spontaneous reaction.
- Point 2: The zinc electrode immersed in the electrolyte solution is undergoing oxidation. The half-reaction is Zn(s) → Zn²⁺(aq) + 2e⁻.
- Point 3: Zinc ions (Zn²⁺) are moving through the electrolyte solution from the zinc electrode towards the copper electrode.
- Point 4: Copper ions from the solution are being reduced at the copper electrode. The half-reaction is Cu²⁺(aq) + 2e⁻ → Cu(s).
Answer for screen readers
Here are the descriptions for the requested points:
- Point 1: It's a power supply providing voltage, moving electrons from the zinc electrode to the copper electrode, enabling the non-spontaneous reaction.
- Point 2: The zinc electrode immersed in the electrolyte solution is undergoing oxidation. The half-reaction is Zn(s) → Zn²⁺(aq) + 2e⁻.
- Point 3: Zinc ions (Zn²⁺) are moving through the electrolyte solution from the zinc electrode towards the copper electrode.
- Point 4: Copper ions from the solution are being reduced at the copper electrode. The half-reaction is Cu²⁺(aq) + 2e⁻ → Cu(s).
More Information
In an electrolytic cell, a non-spontaneous reaction is driven by an external power source. Oxidation occurs at the anode (zinc electrode in this case), and reduction occurs at the cathode (copper electrode).
Tips
A common mistake is confusing electrolytic cells with galvanic cells. Electrolytic cells require an external power source, while galvanic cells produce electricity through spontaneous reactions.
Sources
- Electrolytic Cells - Chemistry LibreTexts - chem.libretexts.org
- Electrolytic Cell - Definition, Diagram, Working, Applications, FAQs - byjus.com
- 17.7 Electrolysis | General College Chemistry II - Lumen Learning - courses.lumenlearning.com
AI-generated content may contain errors. Please verify critical information