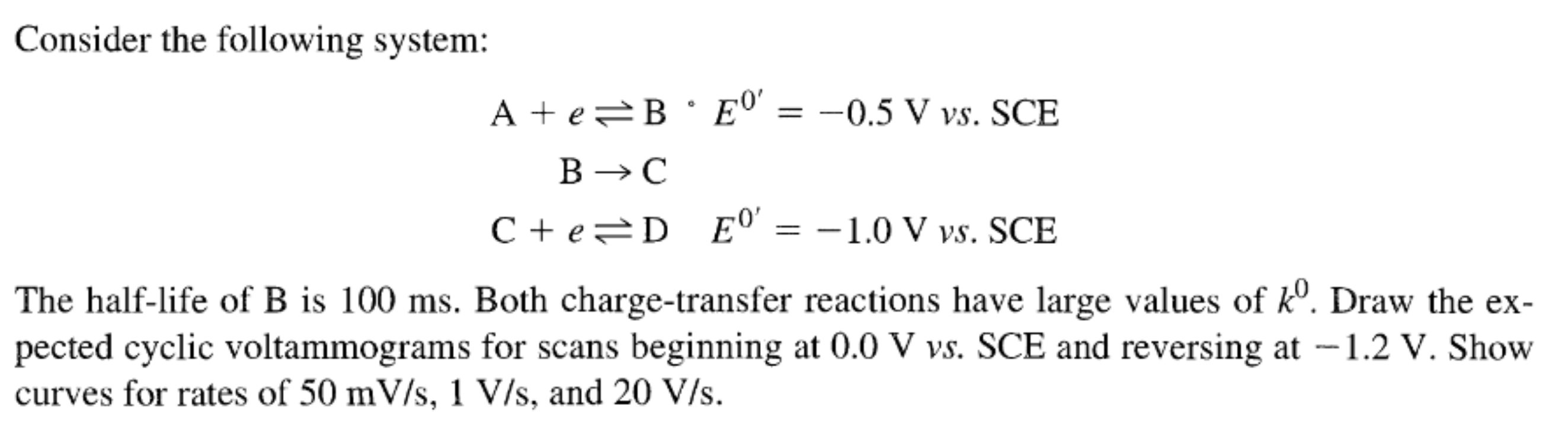
Consider the following system: A + e- ⇌ B E0' = -0.5 V vs. SCE B -> C C + e- ⇌ D E0' = -1.0 V vs. SCE The half-life of B is 100 ms. Both charge-transfer reactions have large va... Consider the following system: A + e- ⇌ B E0' = -0.5 V vs. SCE B -> C C + e- ⇌ D E0' = -1.0 V vs. SCE The half-life of B is 100 ms. Both charge-transfer reactions have large values of k0. Draw the expected cyclic voltammograms for scans beginning at 0.0 V vs. SCE and reversing at -1.2 V. Show curves for rates of 50 mV/s, 1 V/s, and 20 V/s.
Understand the Problem
The question requires sketching cyclic voltammograms for a given electrochemical system at different scan rates. The system involves two reversible electron transfer reactions (A + e- ⇌ B and C + e- ⇌ D) with large rate constants, and an irreversible chemical reaction (B → C) with a half-life of 100 ms. The task involves illustrating how the scan rate affects the shapes and positions of the voltammograms, especially considering the kinetics of the reactions.
Answer
The requested cyclic voltammograms are described above, showing the impact of the $B \rightarrow C$ reaction at different scan rates on the observed peak currents for the A/B and C/D redox couples.
Answer for screen readers
The sketch of the cyclic voltammograms cannot be rendered in text, but the description is as follows:
-
50 mV/s: A prominent reduction peak at -0.5 V (A to B) with a very small or nearly absent oxidation peak on the reverse scan (B to A). A more significant reduction peak at -1.0 V (C to D), with a corresponding oxidation peak on the reverse scan.
-
1 V/s: A reduction peak at -0.5 V (A to B) with a noticeable but smaller oxidation peak on the reverse scan (B to A). A smaller reduction peak at -1.0 V (C to D) compared to the 50 mV/s scan, with a corresponding oxidation peak on the reverse scan.
-
20 V/s: A well-defined reduction peak at -0.5 V (A to B) with a nearly symmetrical oxidation peak on the reverse scan (B to A), indicating a reversible couple. Minimal impact on the C/D redox couple at -1.0 V compared to the other rates.
Steps to Solve
- Understanding the Electrochemical System
The system has two reversible electrochemical reactions (A/B and C/D) and one irreversible chemical reaction (B to C). The standard reduction potentials ($E^{0'}$) for A/B and C/D are -0.5 V and -1.0 V vs. SCE, respectively. The forward rate constants ($k^{0}$) for both redox couples are large, implying rapid electron transfer. The half-life of species B converting to C is 100 ms, meaning the kinetics of this conversion will influence the cyclic voltammograms, particularly at slower scan rates.
- Analyzing the Scan Rates
We need to consider three different scan rates: 50 mV/s, 1 V/s, and 20 V/s. Each scan starts at 0.0 V and reverses at -1.2 V. The scan rate will dictate how much of species B converts to C during the time of the experiment
- Cyclic Voltammetry at 50 mV/s
At a slow scan rate (50 mV/s), the experiment timescale is long. This gives species B ample time to convert to C via the chemical reaction $B \rightarrow C$. As the potential is scanned negatively from 0.0 V, a reduction peak for A to B will be observed near -0.5 V. However, due to the slow scan rate, most of the generated B will convert to C before the reverse scan. During the reverse scan (positive direction), the oxidation peak of B back to A will be significantly diminished because B has largely been consumed. Instead, because B converts to C, and C is reduced to D, a prominent reduction of C to D will occur at $E^{0'}$ = -1.0 V. The oxidation peak of D will be observed on the reverse scan. So we can expect to see peaks around -0.5 V and -1.0 V. The peak at -0.5 V will have a small return peak, and should diminish over time.
- Cyclic Voltammetry at 1 V/s
At an intermediate scan rate (1 V/s), the timescale is shorter than the 50 mV/s case, but still allows a fraction of B to convert to C. As the potential is scanned negatively, the reduction peak for A to B emerges at -0.5 V. Now, a smaller but noticeable amount of B has time to convert to C. Thus, on the reverse scan, the oxidation peak for B back to A will be more significant compared to the 50 mV/s scan but still smaller than we'd expect for a fully reversible couple. We also observe the reduction of C to D near -1.0 V, though it's less pronounced than in the 50 mV/s case because less B has had time to convert to C. Because the reaction is reversible, we will also see the oxidation peak of D.
- Cyclic Voltammetry at 20 V/s
At a fast scan rate (20 V/s), the experiment occurs quickly, minimizing the amount of B that can convert to C. The reduction peak for A to B will appear at -0.5 V. Because the scan is fast, only a minimal amount of B converts to C meaning on the reverse scan, a prominent oxidation peak for B back to A will be observed. The reduction and oxidation peaks for the A/B couple will be nearly symmetrical, indicating a reversible process. The effect on the C/D couple will be small.
- Sketching the Voltammograms
Based on the above analysis, we can sketch the cyclic voltammograms. The x-axis is the potential (V vs. SCE), and the y-axis is the current. The positioning and size of the peaks for the electrochemical reactions A + e- ⇌ B and C + e- ⇌ D need to be illustrated according to the specified rates.
The sketch of the cyclic voltammograms cannot be rendered in text, but the description is as follows:
-
50 mV/s: A prominent reduction peak at -0.5 V (A to B) with a very small or nearly absent oxidation peak on the reverse scan (B to A). A more significant reduction peak at -1.0 V (C to D), with a corresponding oxidation peak on the reverse scan.
-
1 V/s: A reduction peak at -0.5 V (A to B) with a noticeable but smaller oxidation peak on the reverse scan (B to A). A smaller reduction peak at -1.0 V (C to D) compared to the 50 mV/s scan, with a corresponding oxidation peak on the reverse scan.
-
20 V/s: A well-defined reduction peak at -0.5 V (A to B) with a nearly symmetrical oxidation peak on the reverse scan (B to A), indicating a reversible couple. Minimal impact on the C/D redox couple at -1.0 V compared to the other rates.
More Information
Cyclic voltammetry is a powerful technique used to study the redox properties of chemical species. By varying the scan rate, one can probe kinetic and mechanistic aspects of electrochemical reactions. The faster the scan rate, the less time available for chemical reactions coupled to the electron transfer to occur.
Tips
- Forgetting to consider the impact of the chemical reaction B → C on the concentrations of B and C at different scan rates.
- Not recognizing that faster scan rates mean less time for the chemical reaction to occur, leading to more reversible behavior for the A/B couple.
- Incorrectly positioning the reduction and oxidation peaks relative to the standard reduction potentials.
- Not showing a decrease of the reverse peak at -0.5V and increase of the forward peak at -1.0V with decreasing scan rate.
AI-generated content may contain errors. Please verify critical information