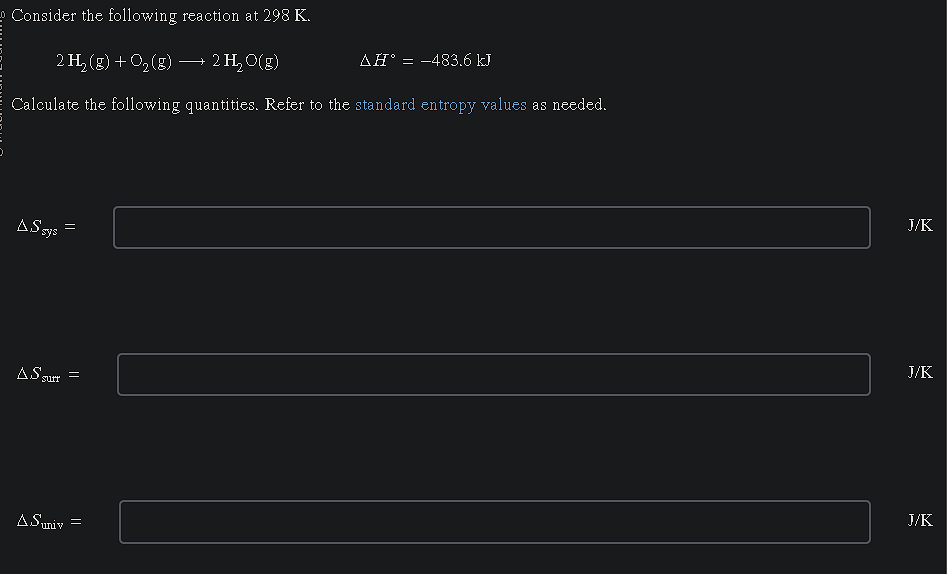
Consider the following reaction at 298 K. 2 H2(g) + O2(g) → 2 H2O(g) ΔH° = -483.6 kJ Calculate the following quantities. ΔS_sys = , ΔS_surr = , ΔS_univ =
Understand the Problem
The question is asking to calculate the entropy changes for the system (ΔS_sys), surroundings (ΔS_surr), and the universe (ΔS_univ) for the given chemical reaction at 298 K, using standard entropy values as needed.
Answer
$$ \Delta S_{sys} = -88.6 \, \text{J/K}, \Delta S_{surr} = 1622.15 \, \text{J/K}, \Delta S_{univ} = 1533.55 \, \text{J/K} $$
Answer for screen readers
$$ \Delta S_{sys} = -88.6 , \text{J/K} $$
$$ \Delta S_{surr} = 1622.15 , \text{J/K} $$
$$ \Delta S_{univ} = 1533.55 , \text{J/K} $$
Steps to Solve
- Calculate ΔS_sys (System Entropy Change)
To find the entropy change of the system ($\Delta S_{sys}$), use the standard entropy values for the reactants and products. The formula is:
$$ \Delta S_{sys} = \sum S^\circ_{products} - \sum S^\circ_{reactants} $$
From standard entropy tables, assume the following values (in J/K·mol):
- $S^\circ(H_2(g)) = 130.6$
- $S^\circ(O_2(g)) = 205.0$
- $S^\circ(H_2O(g)) = 188.8$
For the reaction:
$$ \Delta S_{sys} = [2 \cdot S^\circ(H_2O)] - [2 \cdot S^\circ(H_2) + S^\circ(O_2)] $$
- Plug in the values
Now substituting in the values:
$$ \Delta S_{sys} = [2 \cdot 188.8] - [2 \cdot 130.6 + 205.0] $$
Calculating it:
$$ \Delta S_{sys} = 377.6 - (261.2 + 205.0) $$ $$ \Delta S_{sys} = 377.6 - 466.2 $$ $$ \Delta S_{sys} = -88.6 , \text{J/K} $$
- Calculate ΔS_surr (Surroundings Entropy Change)
The entropy change of the surroundings ($\Delta S_{surr}$) can be calculated using the enthalpy change ($\Delta H^\circ$) and the temperature (T):
$$ \Delta S_{surr} = -\frac{\Delta H^\circ}{T} $$
Substituting the values:
$$ \Delta S_{surr} = -\frac{-483600 , \text{J}}{298 , \text{K}} $$
- Simplify the equation
Calculating:
$$ \Delta S_{surr} = \frac{483600}{298} = 1622.15 , \text{J/K} $$
- Calculate ΔS_univ (Total Entropy Change)
The total entropy change of the universe ($\Delta S_{univ}$) is the sum of $\Delta S_{sys}$ and $\Delta S_{surr}$:
$$ \Delta S_{univ} = \Delta S_{sys} + \Delta S_{surr} $$
Substituting in:
$$ \Delta S_{univ} = -88.6 + 1622.15 $$
- Final Calculation
Calculating the total:
$$ \Delta S_{univ} = 1533.55 , \text{J/K} $$
$$ \Delta S_{sys} = -88.6 , \text{J/K} $$
$$ \Delta S_{surr} = 1622.15 , \text{J/K} $$
$$ \Delta S_{univ} = 1533.55 , \text{J/K} $$
More Information
The calculations show the contributions of the system and surroundings to the total entropy change. A negative $\Delta S_{sys}$ indicates the system becomes more ordered, while a positive $\Delta S_{surr}$ signifies that the surroundings gain disorder from the exothermic reaction.
Tips
- Incorrect Sign for ΔH: Ensure the enthalpy change is used correctly since it's negative for exothermic reactions.
- Units Confusion: Always keep track of units (Joules vs. kilojoules) when using standard entropy values and converting ΔH.
AI-generated content may contain errors. Please verify critical information