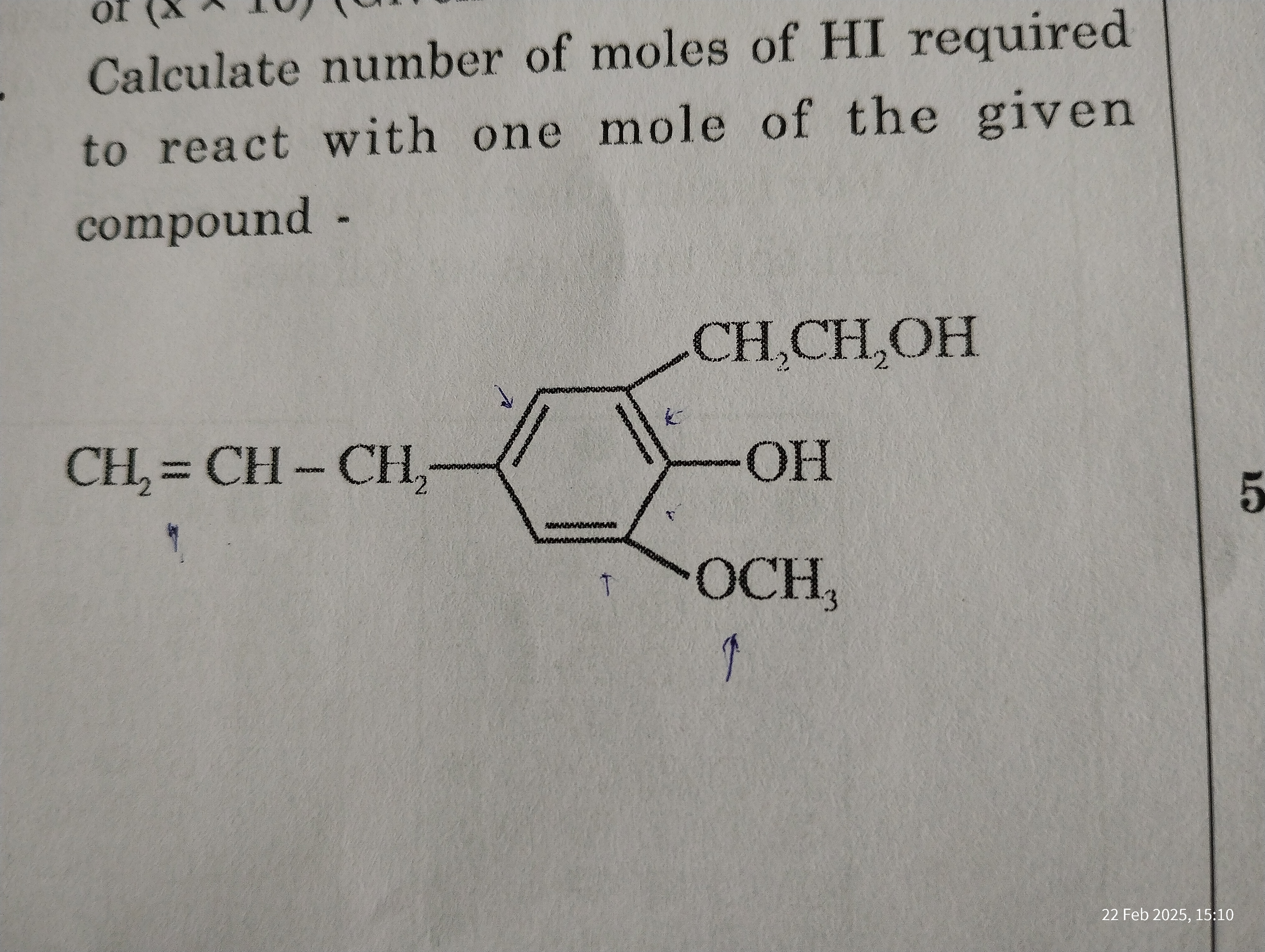
Calculate the number of moles of HI required to react with one mole of the given compound.
Understand the Problem
The question asks to determine the number of moles of HI (hydrogen iodide) needed to react with one mole of the given organic compound. The compound has multiple functional groups that can react with HI, including an alkene, a phenol, an alcohol, and an ether.
Answer
3
Answer for screen readers
3
Steps to Solve
- Identify the HI-reactive functional groups
The given compound contains the following functional groups that can react with HI:
- An alkene ($CH_2=CH-$)
- A phenol (-OH directly attached to benzene ring)
- An alcohol ($-CH_2CH_2OH$)
- An ether ($-OCH_3$)
- Determine the reaction of HI with the alkene
The alkene reacts with HI via an addition reaction. One mole of HI is consumed per mole of alkene. $CH_2=CH-CH_2- + HI \rightarrow CH_3-CHI-CH_2-$
- Determine the reaction of HI with the alcohol
The alcohol reacts with HI to form an alkyl halide and water. One mole of HI is consumed per mole of alcohol. $-CH_2CH_2OH + HI \rightarrow -CH_2CH_2I + H_2O$
- Determine the reaction of HI with the ether
The ether reacts with HI to form an alcohol and an alkyl iodide. One mole of HI is consumed per mole of ether. $R-O-R' + HI \rightarrow R-OH + R'-I$ In this case, $-OCH_3 + HI \rightarrow -OH + CH_3I$, so the methoxy group turns into a phenol group.
- Determine the reaction of HI with the phenol
The phenol does not react with HI under normal conditions because breaking the C-O bond requires very harsh conditions.
- Total HI moles required
Sum up the moles of HI required for each reactive functional group:
- Alkene: 1 mole
- Alcohol: 1 mole
- Ether: 1 mole
Total moles of HI = 1 (alkene) + 1 (alcohol) + 1 (ether) = 3 moles
3
More Information
HI will react with the alkene, alcohol, and ether groups, each consuming one mole of HI. The phenol group will not react under normal conditions.
Tips
A common mistake is assuming that phenols react with HI similar to alcohols or ethers under normal conditions. Phenols require very harsh conditions to react with HI.
AI-generated content may contain errors. Please verify critical information