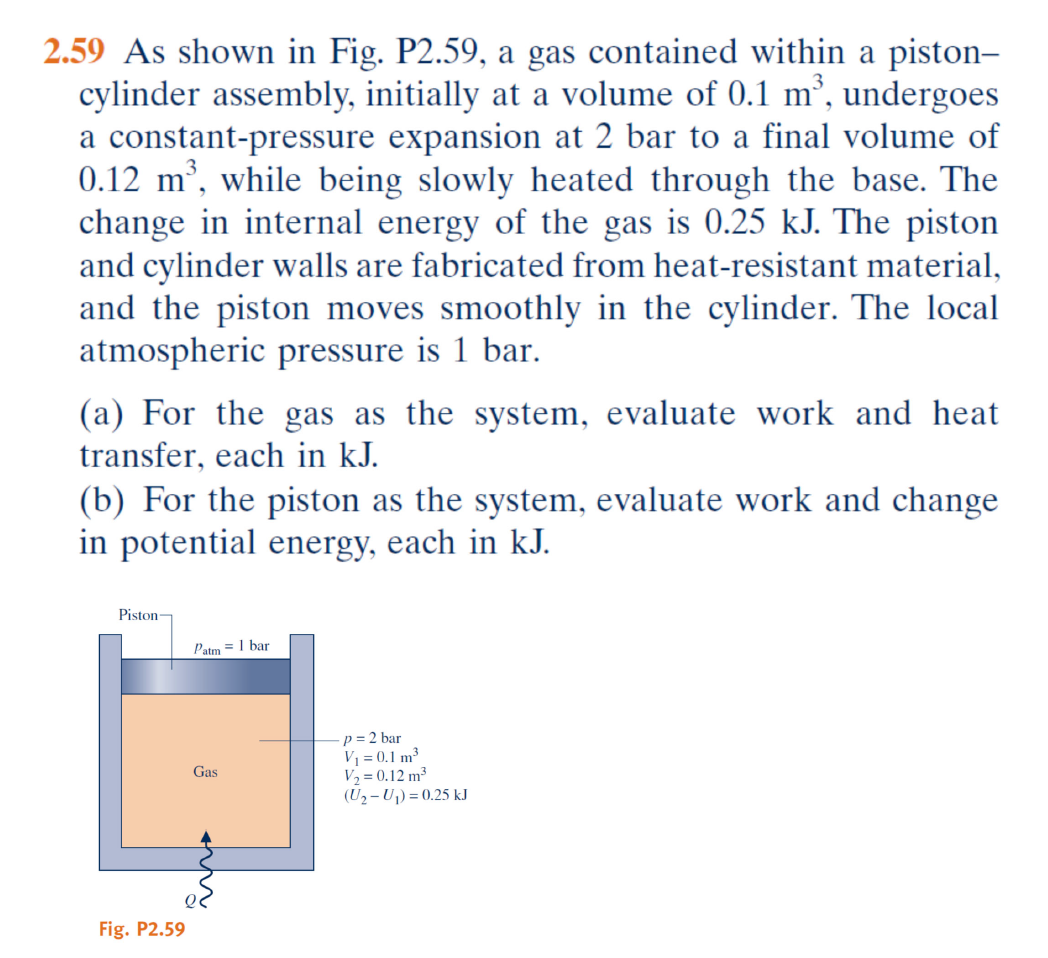
As shown in Fig. P2.59, a gas contained within a piston-cylinder assembly, initially at a volume of 0.1 m³, undergoes a constant-pressure expansion at 2 bar to a final volume of 0.... As shown in Fig. P2.59, a gas contained within a piston-cylinder assembly, initially at a volume of 0.1 m³, undergoes a constant-pressure expansion at 2 bar to a final volume of 0.12 m³, while being slowly heated through the base. The change in internal energy of the gas is 0.25 kJ. The local atmospheric pressure is 1 bar. (a) For the gas as the system, evaluate work and heat transfer, each in kJ. (b) For the piston as the system, evaluate work and change in potential energy, each in kJ.
Understand the Problem
The question is asking us to evaluate the work and heat transfer for a gas expanding in a piston-cylinder system, as well as the work and change in potential energy for the piston. This involves applying thermodynamic principles related to work done during expansion, heat transfer, and energy changes in the system.
Answer
(a) $4 \, \text{kJ}$, $4.25 \, \text{kJ}$; (b) $2 \, \text{kJ}$, $0 \, \text{kJ}$
Answer for screen readers
(a) Work done by the gas: $4 , \text{kJ}$, Heat transfer: $4.25 , \text{kJ}$
(b) Work done on the piston: $2 , \text{kJ}$, Change in potential energy: $0 , \text{kJ}$
Steps to Solve
- Calculate Work for the Gas as the System
The work done by the gas during expansion at constant pressure can be calculated using the formula: $$ W = P \cdot \Delta V $$ where:
- $P = 2 \text{ bar} = 200 \text{ kPa}$ (since 1 bar = 100 kPa)
- $\Delta V = V_2 - V_1 = 0.12 , \text{m}^3 - 0.1 , \text{m}^3 = 0.02 , \text{m}^3$
Substituting the values: $$ W = 200 , \text{kPa} \times 0.02 , \text{m}^3 = 4 , \text{kJ} $$
- Determine Heat Transfer for the Gas as the System
Using the first law of thermodynamics: $$ \Delta U = Q - W $$ where:
- $\Delta U = 0.25 , \text{kJ}$
- $W = 4 , \text{kJ}$
Rearranging to find heat transfer $Q$: $$ Q = \Delta U + W = 0.25 , \text{kJ} + 4 , \text{kJ} = 4.25 , \text{kJ} $$
- Calculate Work for the Piston as the System
For the piston, the work done on the piston can be considered as the work done against the atmospheric pressure: $$ W_{piston} = P_{atm} \cdot \Delta V = 1 \text{ bar} \cdot (V_2 - V_1) $$ Substituting values: $$ W_{piston} = 100 , \text{kPa} \times 0.02 , \text{m}^3 = 2 , \text{kJ} $$
- Evaluate Change in Potential Energy for the Piston
Since specific details about the height change are not given here, we assume no significant change in height which leads to: $$ \Delta PE = 0 , \text{kJ} $$
In a typical case, if height change were provided, we would use: $$ \Delta PE = m \cdot g \cdot h $$ But in this scenario, we state that potential energy change can be approximated as zero.
(a) Work done by the gas: $4 , \text{kJ}$, Heat transfer: $4.25 , \text{kJ}$
(b) Work done on the piston: $2 , \text{kJ}$, Change in potential energy: $0 , \text{kJ}$
More Information
The calculations above apply basic principles of thermodynamics, particularly the first law, to evaluate work done during a gas expansion and its heat transfer. Understanding how work and energy interact in a system is crucial in thermodynamics.
Tips
- Confusing the sign of work: Make sure to remember if work is done by the system (gas) or on the system (piston).
- Ignoring units conversion: Make sure to convert pressure from bars to kPa where necessary to maintain consistency.
AI-generated content may contain errors. Please verify critical information