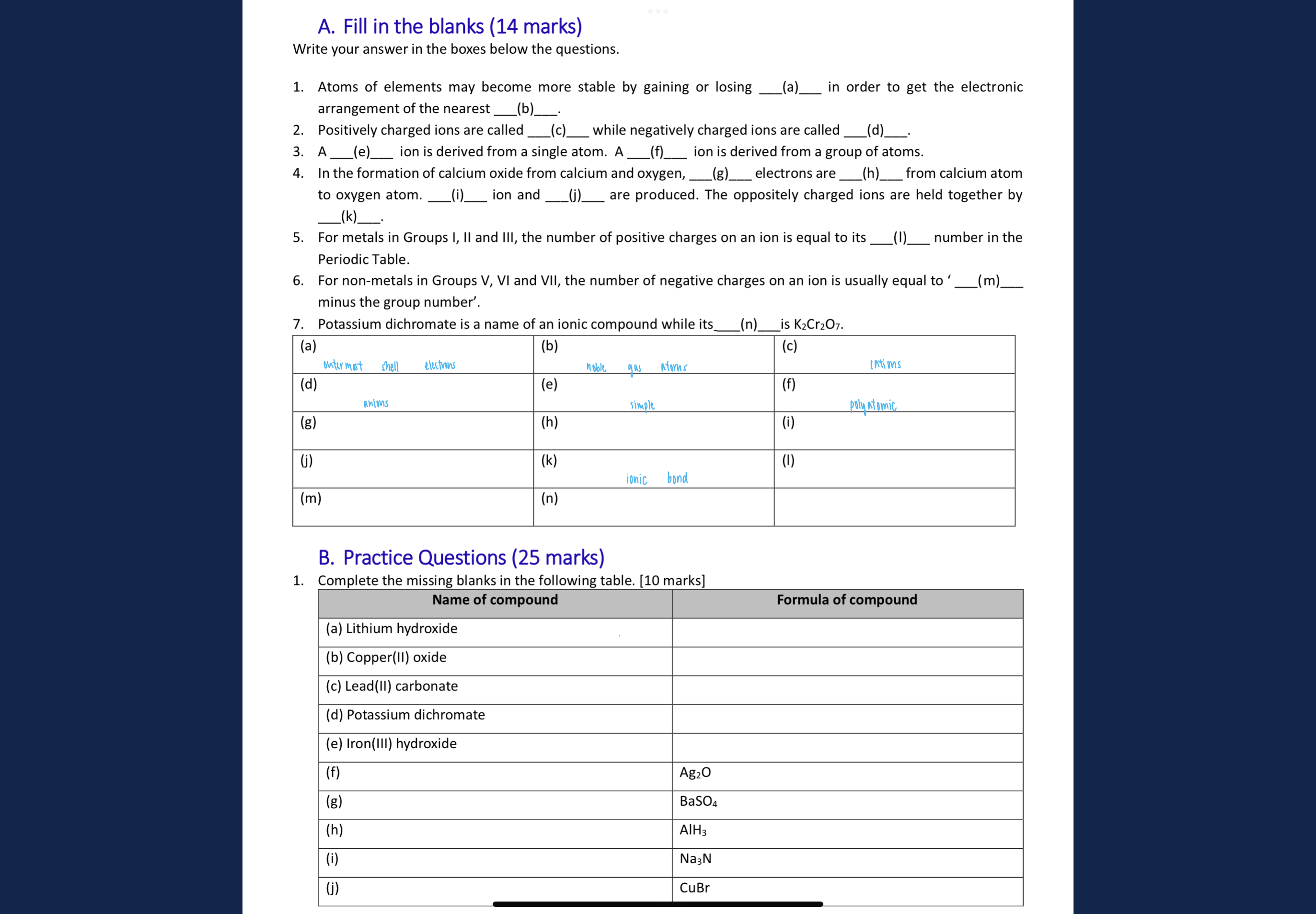
A. Fill in the blanks. Write your answer in the boxes below the questions. 1. Atoms of elements may become more stable by gaining or losing ___ in order to get the electronic arran... A. Fill in the blanks. Write your answer in the boxes below the questions. 1. Atoms of elements may become more stable by gaining or losing ___ in order to get the electronic arrangement of the nearest ___. 2. Positively charged ions are called ___ while negatively charged ions are called ___. 3. A ___ ion is derived from a single atom. A ___ ion is derived from a group of atoms. 4. In the formation of calcium oxide from calcium and oxygen, ___ electrons are ___ from calcium atom to oxygen atom. ___ ion and ___ ion are produced. The oppositely charged ions are held together by ___. 5. For metals in Groups I, II and III, the number of positive charges on an ion is equal to its ___ number in the Periodic Table. 6. For non-metals in Groups V, VI and VII, the number of negative charges on an ion is usually equal to '___ minus the group number'. 7. Potassium dichromate is a name of an ionic compound while its ___ is K2Cr2O7. B. Practice Questions. Complete the missing blanks in the following table. Name of compound: (a) Lithium hydroxide, (b) Copper(II) oxide, (c) Lead(II) carbonate, (d) Potassium dichromate, (e) Iron(III) hydroxide, (f), (g), (h), (i), (j)
Understand the Problem
The question is asking to fill in the blanks related to chemistry concepts such as the stability of atoms, types of ions, the formation of compounds, and specific ionic compounds. It is also requesting to complete a table with names and formulas of compounds.
Answer
1. (a) Electrons, (b) Noble gas, (c) Cations, (d) Anions, (e) Simple, (f) Polyatomic, (g) Two, (h) Transferred, (i) Calcium, (j) Oxide, (k) Ionic bond, (l) Group, (m) 8, (n) Formula. Practice: LiOH, CuO, PbCO3, K2Cr2O7, Fe(OH)3.
- (a) Electrons, (b) Noble gas, (c) Cations, (d) Anions, (e) Simple, (f) Polyatomic, (g) Two, (h) Transferred, (i) Calcium, (j) Oxide, (k) Ionic bond, (l) Group, (m) 8, (n) Formula. Practice table: (a) LiOH, (b) CuO, (c) PbCO3, (d) K2Cr2O7, (e) Fe(OH)3.
Answer for screen readers
- (a) Electrons, (b) Noble gas, (c) Cations, (d) Anions, (e) Simple, (f) Polyatomic, (g) Two, (h) Transferred, (i) Calcium, (j) Oxide, (k) Ionic bond, (l) Group, (m) 8, (n) Formula. Practice table: (a) LiOH, (b) CuO, (c) PbCO3, (d) K2Cr2O7, (e) Fe(OH)3.
More Information
Atoms gain or lose electrons to achieve a stable electronic arrangement similar to noble gases. Ionic bonds form between ions of opposite charges. Metals typically lose electrons to form positive ions while non-metals gain electrons to form negative ions.
Tips
Common mistake is confusing cations with anions. Remember, cations are positive; anions are negative.
Sources
- Regents Chemistry Topics Review Packet - oleanschools.org
AI-generated content may contain errors. Please verify critical information