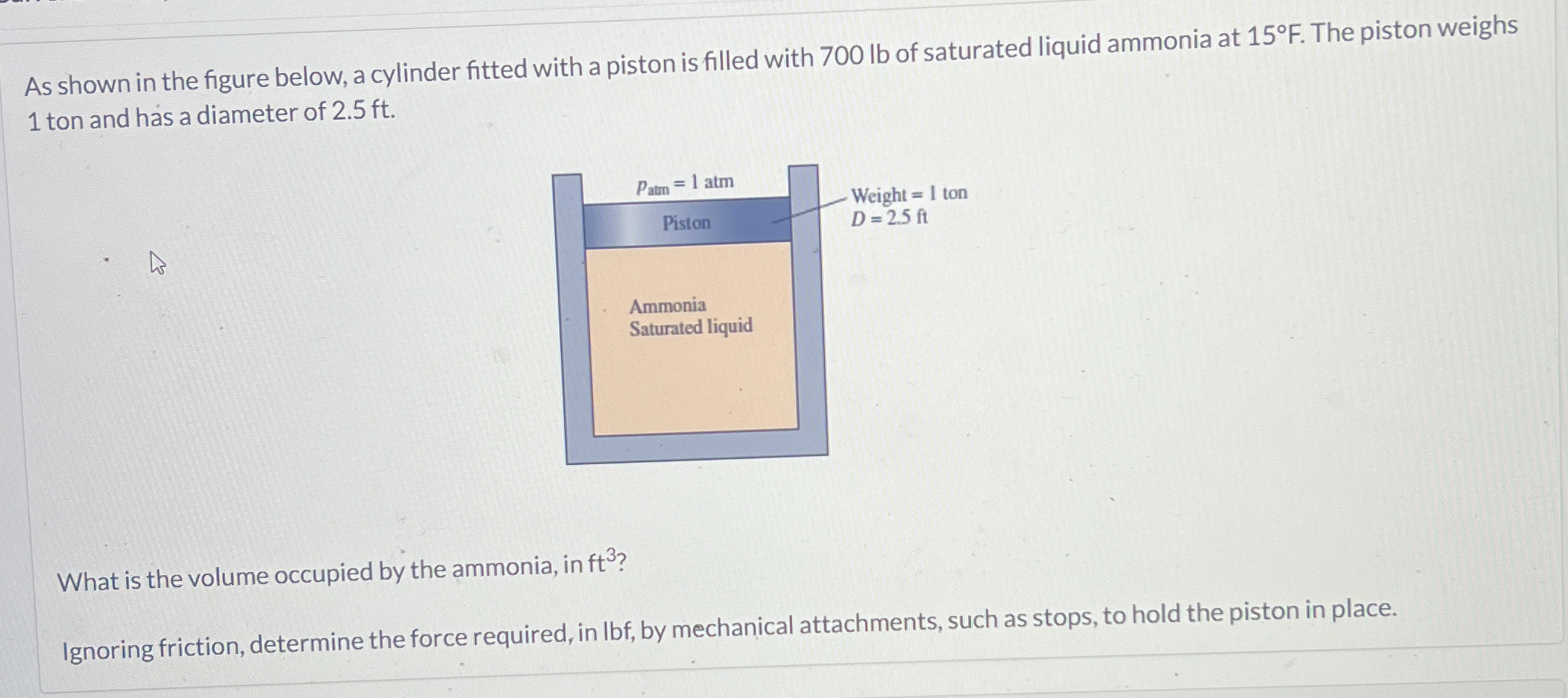
A cylinder fitted with a piston is filled with 700 lb of saturated liquid ammonia at 15°F. The piston weighs 1 ton and has a diameter of 2.5 ft. What is the volume occupied by the... A cylinder fitted with a piston is filled with 700 lb of saturated liquid ammonia at 15°F. The piston weighs 1 ton and has a diameter of 2.5 ft. What is the volume occupied by the ammonia, in ft³? Ignoring friction, determine the force required, in lbf, by mechanical attachments, such as stops, to hold the piston in place.
Understand the Problem
The problem describes a cylinder fitted with a piston containing 700 lb of saturated liquid ammonia at 15°F. The piston weighs 1 ton and has a diameter of 2.5 ft. The problem asks to calculate: 1) the volume occupied by the ammonia in ft³ and 2) the force required, in lbf, to hold the piston in place, ignoring friction.
Answer
The volume is $17.885 \ ft^3$ and the force required is $12388.16 \ lbf$.
Answer for screen readers
The volume occupied by the ammonia is $17.885 \ ft^3$. The force required to hold the piston in place is $12388.16 \ lbf$.
Steps to Solve
- Find the specific volume of saturated liquid ammonia at 15°F
From thermodynamics tables, the specific volume of saturated liquid ammonia at 15°F is $v_f = 0.02555 \ ft^3/lb$.
- Calculate the volume occupied by the ammonia
The volume $V$ is the product of the mass $m$ and the specific volume $v_f$: $ V = m \times v_f $ $ V = 700 \ lb \times 0.02555 \ ft^3/lb = 17.885 \ ft^3 $
- Calculate the area of the piston
The area $A$ of the piston is: $A = \pi r^2 = \pi (\frac{D}{2})^2 = \pi (\frac{2.5 \ ft}{2})^2 = 4.9087 \ ft^2 $
- Calculate the weight of the piston
The piston weighs 1 ton, which is 2000 lb. So, $W_{piston} = 2000 \ lb$.
- Calculate the force due to atmospheric pressure
The atmospheric pressure is $P_{atm} = 1 \ atm = 14.696 \ psi = 14.696 \ lb/in^2$. We need to convert the area to $in^2$:
$A = 4.9087 \ ft^2 \times (12 \ in/ft)^2 = 706.8672 \ in^2 $
The force due to atmospheric pressure $F_{atm} = P_{atm} \times A = 14.696 \ lb/in^2 \times 706.8672 \ in^2 = 10388.16 \ lb$
- Calculate the total downward force
The total downward force $F_{down}$ exerted on the ammonia is the sum of the piston weight and the atmospheric force: $F_{down} = W_{piston} + F_{atm} = 2000 \ lb + 10388.16 \ lb = 12388.16 \ lb$
- Determine the force required to hold the piston in place
Since we want to find the force required to hold the piston in place and we know the forces acting downwards, we can say that the mechanical attachments must exert an upward force equal to the net downward force. Since the pressure exerted by the ammonia is acting upwards. Then the force required $F_{req}$ to hold the piston in place is equal to the total downward force. $F_{req} = 12388.16 \ lb$
The volume occupied by the ammonia is $17.885 \ ft^3$. The force required to hold the piston in place is $12388.16 \ lbf$.
More Information
Ammonia is commonly used as a refrigerant in industrial applications due to its thermodynamic properties.
Tips
- Forgetting to convert units (e.g., tons to pounds, $ft^2$ to $in^2$)
- Not including atmospheric pressure when calculating forces on the piston.
- Using the wrong thermodynamic properties for ammonia.
AI-generated content may contain errors. Please verify critical information