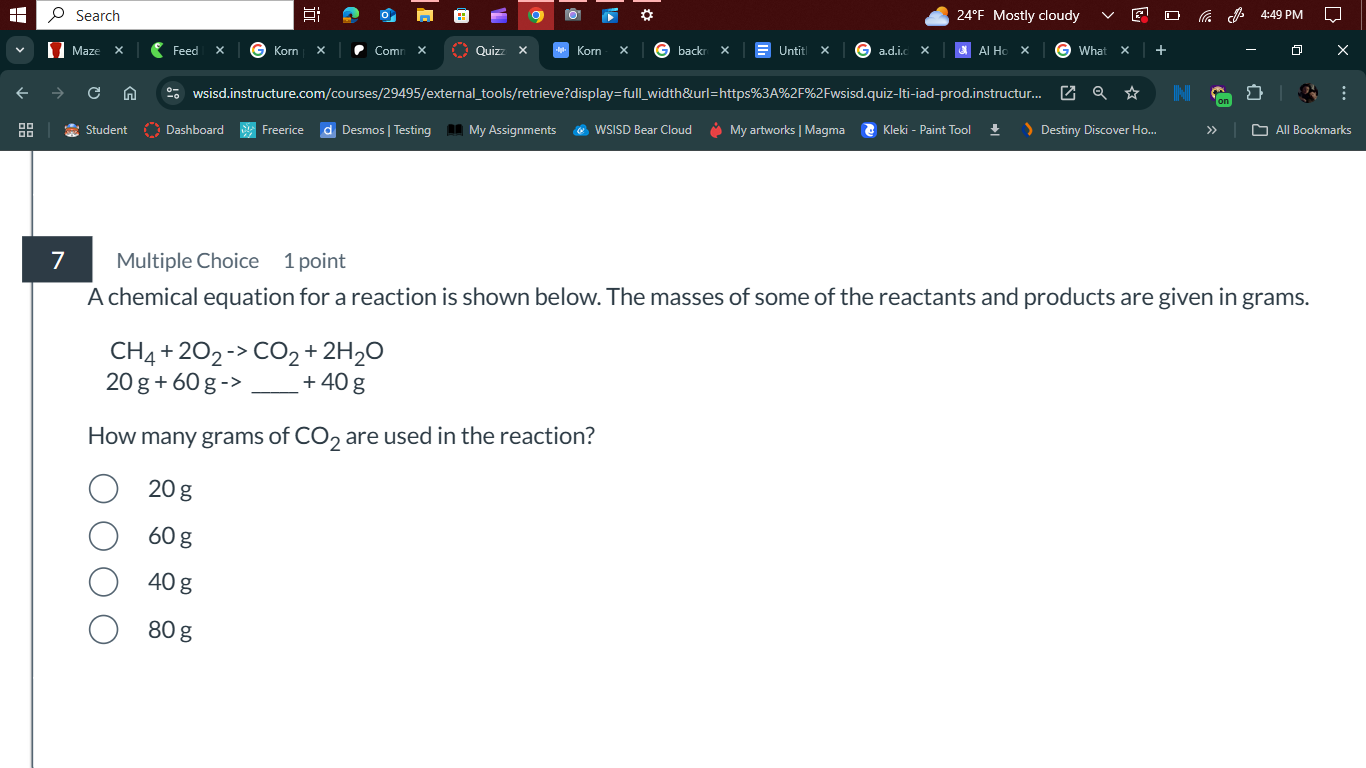
A chemical equation for a reaction is shown below. The masses of some of the reactants and products are given in grams. $CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$, $20g + 60g \rightarr... A chemical equation for a reaction is shown below. The masses of some of the reactants and products are given in grams. $CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$, $20g + 60g \rightarrow \_\_\_ + 40g$. How many grams of $CO_2$ are used in the reaction?
Understand the Problem
The problem is asking to find the amount of $CO_2$ produced in a chemical reaction given the amounts of reactants and one of the products. We can solve this by applying the law of conservation of of mass, which says that matter cannot be created or destroyed.
Answer
$40$ g
Answer for screen readers
$40$ g of $CO_2$ are used in the reaction.
Steps to Solve
- Determine the total mass of the reactants
We are given that the reactants are $CH_4$ with a mass of 20g and $2O_2$ with a mass of 60g. The total mass of reactants is: $20g + 60g = 80g$
- Determine the unknown mass of $CO_2$
We know that the total mass of the reactants must equal the total mass of the products. We can set up the following equation: $Mass_{reactants} = Mass_{products}$ $80g = Mass_{CO_2} + 40g$
- Solve for $Mass_{CO_2}$
Subtract 40g from both sides: $80g - 40g = Mass_{CO_2}$ $Mass_{CO_2} = 40g$
$40$ g of $CO_2$ are used in the reaction.
More Information
The law of conservation of mass is a fundamental concept in chemistry, stating that mass is neither created nor destroyed in a closed system.
Tips
A common mistake is to not account for all the reactants or products when applying the law of conservation of mass. Make sure to include all masses on both sides of the equation.
AI-generated content may contain errors. Please verify critical information