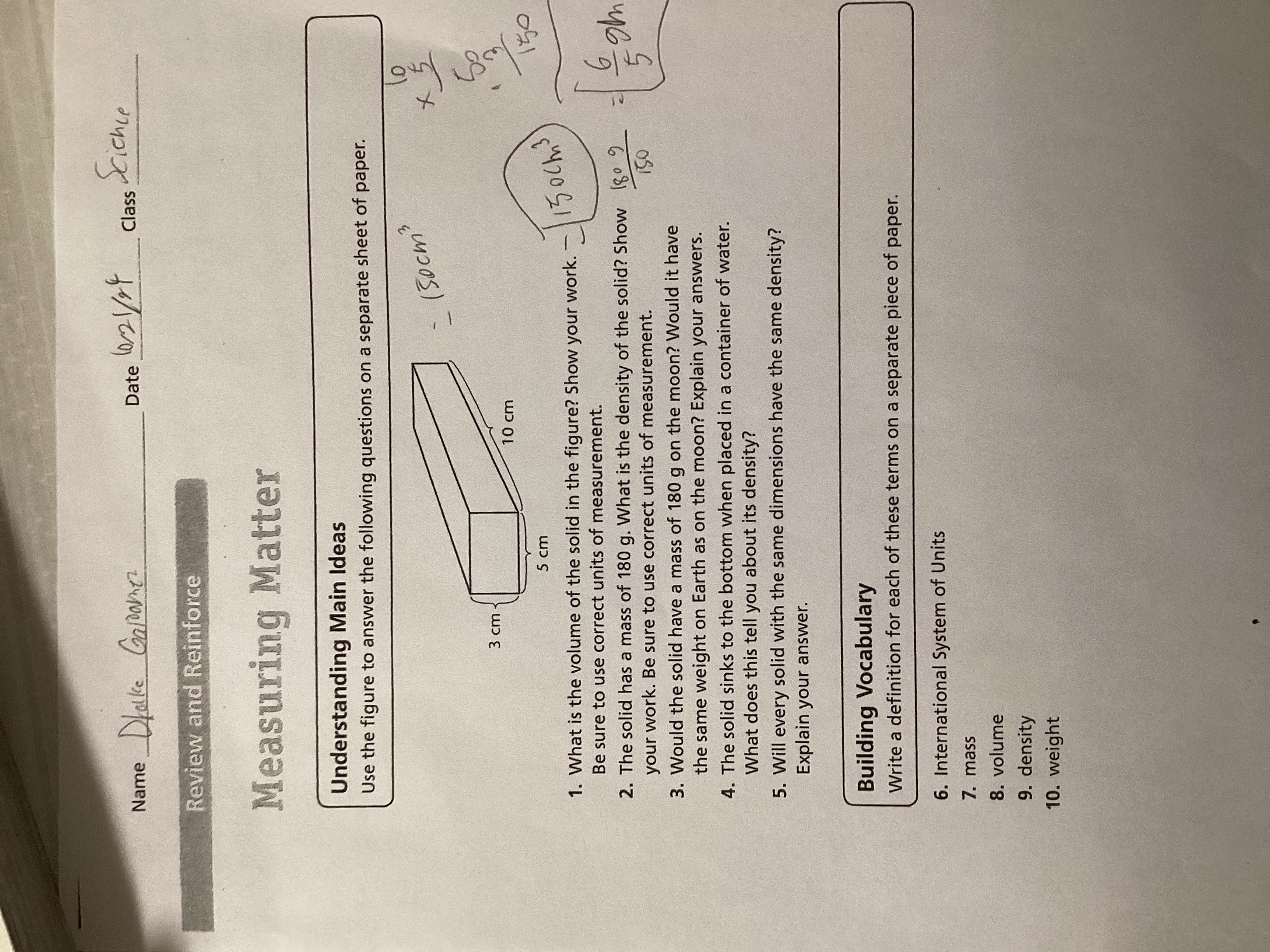
1. What is the volume of the solid in the figure? Show your work. 2. The solid has a mass of 180 g. What is the density of the solid? Show your work. 3. If the solid is placed on t... 1. What is the volume of the solid in the figure? Show your work. 2. The solid has a mass of 180 g. What is the density of the solid? Show your work. 3. If the solid is placed on the moon, would it have the same weight as on Earth? Explain your answer. 4. Will every solid with the same dimensions have the same density? Explain your answer.
Understand the Problem
The question includes several queries related to measuring matter, specifically focusing on volume, mass, and density of a solid figure. It asks for the volume of the solid, the density based on given mass, and further questions about weight on different celestial bodies and the relationship of dimensions and density.
Answer
1. 150 cm³. 2. 1.2 g/cm³. 3. Less weight on the moon. 4. No, it depends on material.
- The volume is 150 cm³. 2. The density is 1.2 g/cm³. 3. The weight would be less on the moon. 4. Solids with the same dimensions do not necessarily have the same density due to material differences.
Answer for screen readers
- The volume is 150 cm³. 2. The density is 1.2 g/cm³. 3. The weight would be less on the moon. 4. Solids with the same dimensions do not necessarily have the same density due to material differences.
More Information
Density is a property of matter that varies with material composition, not just volume. On the moon, the object's weight would be roughly one-sixth of that on Earth due to lower gravity.
Tips
Ensure you multiply correctly when calculating volume and use the correct units when solving for density. Also, remember that density is influenced by material composition.
Sources
AI-generated content may contain errors. Please verify critical information