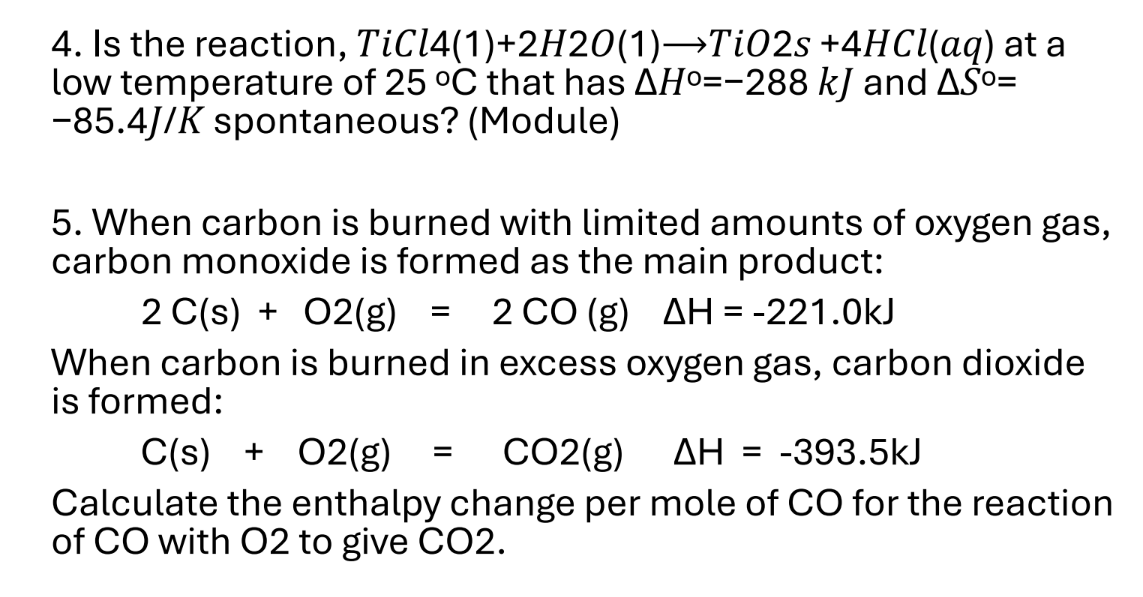
1. Is the reaction, TiCl4(1) + 2H2O(1) → TiO2(s) + 4HCl(aq) at a low temperature of 25°C that has ΔH° = -288 kJ and ΔS° = -85.4 J/K spontaneous? 2. When carbon is burned with lim... 1. Is the reaction, TiCl4(1) + 2H2O(1) → TiO2(s) + 4HCl(aq) at a low temperature of 25°C that has ΔH° = -288 kJ and ΔS° = -85.4 J/K spontaneous? 2. When carbon is burned with limited amounts of oxygen gas, carbon monoxide is formed as the main product: 2 C(s) + O2(g) = 2 CO(g) ΔH = -221.0 kJ When carbon is burned in excess oxygen gas, carbon dioxide is formed: C(s) + O2(g) = CO2(g) ΔH = -393.5 kJ Calculate the enthalpy change per mole of CO for the reaction of CO with O2 to give CO2.
Understand the Problem
The image presents two chemistry questions. The first asks about the spontaneity of a given reaction at a specified temperature, considering its enthalpy and entropy changes. This requires the use of the Gibbs free energy equation (ΔG = ΔH - TΔS) to determine if the reaction is spontaneous (ΔG < 0). The second question involves enthalpy changes in reactions where carbon is burned with different amounts of oxygen. The problem provides two reactions and asks us to calculate the enthalpy change for the reaction of CO with O2 to give CO2. We can use Hess's law to manipulate the given reactions to find the enthalpy change for the target reaction.
Answer
4. Spontaneous 5. $\Delta H = -283.0 \, \text{kJ/mol}$
Answer for screen readers
- The reaction is spontaneous.
- $\Delta H = -283.0 , \text{kJ/mol}$
Steps to Solve
- Calculate $\Delta G$ for the first reaction
To determine spontaneity, we need to calculate the Gibbs Free Energy change ($\Delta G$) using the formula: $\Delta G = \Delta H - T\Delta S$. Given: $\Delta H = -288 , \text{kJ}$ $\Delta S = -85.4 , \text{J/K} = -0.0854 , \text{kJ/K}$ $T = 25^\circ \text{C} = 298 , \text{K}$
$\Delta G = -288 , \text{kJ} - (298 , \text{K})(-0.0854 , \text{kJ/K})$ $\Delta G = -288 , \text{kJ} + 25.4492 , \text{kJ}$ $\Delta G = -262.5508 , \text{kJ}$
- Determine spontaneity based on $\Delta G$
Since $\Delta G < 0$, the reaction is spontaneous at $25^\circ \text{C}$.
- Manipulate the given equations to match the target reaction for the second question
Target reaction: $CO(g) + \frac{1}{2}O_2(g) \rightarrow CO_2(g)$
Given reactions: \begin{align*} 2C(s) + O_2(g) &\rightarrow 2CO(g) \quad \Delta H_1 = -221.0 , \text{kJ} \ C(s) + O_2(g) &\rightarrow CO_2(g) \quad \Delta H_2 = -393.5 , \text{kJ} \end{align*}
- Reverse the first reaction and divide by 2
$CO(g) \rightarrow C(s) + \frac{1}{2}O_2(g) \quad \Delta H' = \frac{221.0}{2} = 110.5 , \text{kJ}$
- Add the modified first reaction to the second reaction
\begin{align*} CO(g) &\rightarrow C(s) + \frac{1}{2}O_2(g) \quad \Delta H' = 110.5 , \text{kJ} \ C(s) + O_2(g) &\rightarrow CO_2(g) \quad \Delta H_2 = -393.5 , \text{kJ} \end{align*} Adding the reactions gives: $CO(g) + C(s) + O_2(g) \rightarrow C(s) + \frac{1}{2}O_2(g) + CO_2(g)$ Simplifying gives the target reaction: $CO(g) + \frac{1}{2}O_2(g) \rightarrow CO_2(g)$
- Calculate the enthalpy change for the target reaction
$\Delta H_{rxn} = \Delta H' + \Delta H_2 = 110.5 , \text{kJ} + (-393.5 , \text{kJ}) = -283.0 , \text{kJ}$
- The reaction is spontaneous.
- $\Delta H = -283.0 , \text{kJ/mol}$
More Information
Hess's Law is based on the fact that enthalpy is a state function. This means that the enthalpy change of a reaction depends only on the initial and final states, not on the path taken.
Tips
For question 4, a common mistake is forgetting to convert the entropy change from J/K to kJ/K before calculating the Gibbs free energy change. Also, make sure that temperature is in Kelvin. For question 5, a mistake is not multiplying or dividing the $\Delta H$ values by the correct factor when manipulating the equations, or not reversing the sign when reversing an equation.
AI-generated content may contain errors. Please verify critical information