1. How many protons does a nitrogen atom have? 2. How many valence electrons does a nitrogen atom have? 3. Is nitrogen reactive or stable? 4. Neon (Ne), which has an atomic number... 1. How many protons does a nitrogen atom have? 2. How many valence electrons does a nitrogen atom have? 3. Is nitrogen reactive or stable? 4. Neon (Ne), which has an atomic number of 10 is in Group 18 in the periodic table. To which group does nitrogen belong? 5. The element directly below nitrogen in the periodic table is phosphorus (P). How many valence electrons does phosphorus have?
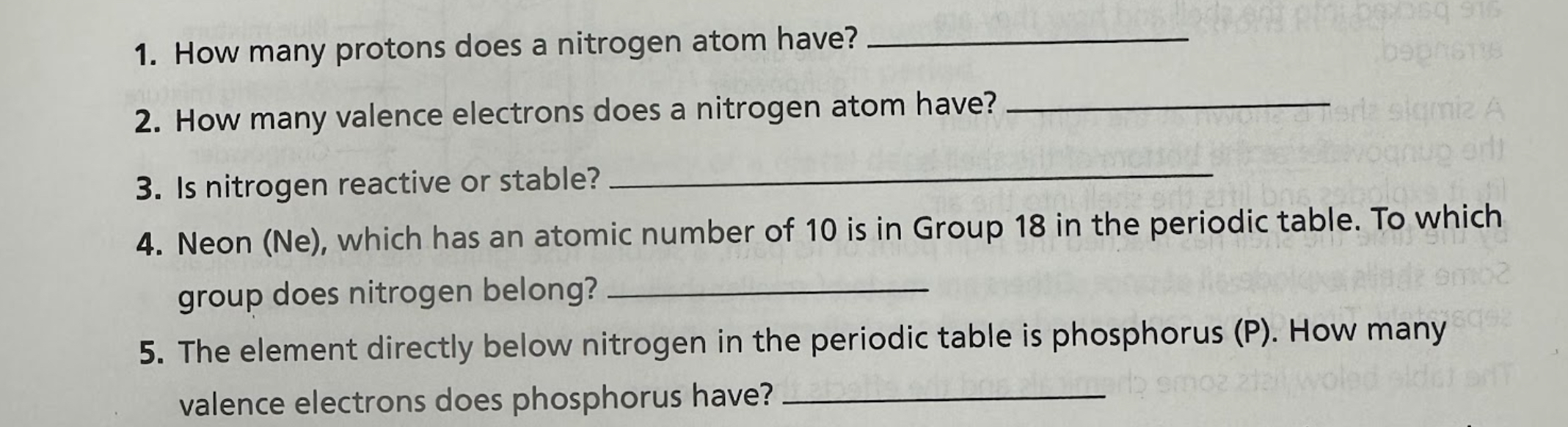
Understand the Problem
The image contains a set of questions related to the properties and characteristics of nitrogen and phosphorus atoms, including their number of protons, valence electrons, reactivity, and group in the periodic table. It is a typical chemistry homework question.
Answer
1. 7 protons; 2. 5 valence electrons; 3. reactive; 4. Group 15; 5. 5 valence electrons
- A nitrogen atom has 7 protons.
- A nitrogen atom has 5 valence electrons.
- Nitrogen is reactive.
- Nitrogen belongs to Group 15.
- Phosphorus has 5 valence electrons.
Answer for screen readers
- A nitrogen atom has 7 protons.
- A nitrogen atom has 5 valence electrons.
- Nitrogen is reactive.
- Nitrogen belongs to Group 15.
- Phosphorus has 5 valence electrons.
More Information
The number of protons determines the element. Valence electrons determine the chemical properties of the element.
Tips
A common mistake is confusing the number of valence electrons with the total number of electrons. Remember valence electrons are the electrons in the outermost shell only.
Sources
AI-generated content may contain errors. Please verify critical information