Podcast
Questions and Answers
What is one reason for studying reaction rates in chemical kinetics?
What is one reason for studying reaction rates in chemical kinetics?
- To determine the molecular weight of reactants
- To identify the colors of reactants
- To calculate the volumes of gases produced
- To better understand reaction mechanisms (correct)
How does increasing the concentration of reactants affect the rate of reaction?
How does increasing the concentration of reactants affect the rate of reaction?
- It increases the frequency of collisions between molecules (correct)
- It leads to a constant reaction rate regardless of concentration
- It decreases the frequency of collisions between molecules
- It has no significant impact on the reaction rate
Which factor is NOT mentioned as affecting the reaction rate?
Which factor is NOT mentioned as affecting the reaction rate?
- Temperature
- Pressure (correct)
- Physical state
- Catalysts
What role do catalysts play in a chemical reaction?
What role do catalysts play in a chemical reaction?
What does collision theory state about the rate of a reaction?
What does collision theory state about the rate of a reaction?
Which rate expression is derived when the slow step is not the first in the mechanism?
Which rate expression is derived when the slow step is not the first in the mechanism?
What indicates that both A and B are involved in the slow step of a reaction mechanism?
What indicates that both A and B are involved in the slow step of a reaction mechanism?
If a mechanism produces an intermediate that cannot be measured directly, what must be done?
If a mechanism produces an intermediate that cannot be measured directly, what must be done?
In the originally presented reaction mechanisms, which mechanism cannot be correct based on the information provided?
In the originally presented reaction mechanisms, which mechanism cannot be correct based on the information provided?
What does the equilibrium constant expression Kc indicate in terms of reactant concentrations?
What does the equilibrium constant expression Kc indicate in terms of reactant concentrations?
What is the overall order of the reaction when the rate law is expressed as rate = k[A][B]?
What is the overall order of the reaction when the rate law is expressed as rate = k[A][B]?
Doubling the concentration of [BrO3-] affects the reaction rate in what way?
Doubling the concentration of [BrO3-] affects the reaction rate in what way?
In the SN1 reaction mechanism, which step is characterized as unimolecular?
In the SN1 reaction mechanism, which step is characterized as unimolecular?
If the rate law is rate = k[B]², what can be concluded about the order with respect to B?
If the rate law is rate = k[B]², what can be concluded about the order with respect to B?
In an SN2 reaction, how does the presence of [OH-] affect the rate of the reaction?
In an SN2 reaction, how does the presence of [OH-] affect the rate of the reaction?
For a reaction governed by rate = k[A], what order reaction is it with respect to A?
For a reaction governed by rate = k[A], what order reaction is it with respect to A?
What determines the rate-determining step in the SN1 reaction mechanism?
What determines the rate-determining step in the SN1 reaction mechanism?
When doubling [H+] results in the reaction rate quadrupling, what does this indicate about its order?
When doubling [H+] results in the reaction rate quadrupling, what does this indicate about its order?
How does pressure affect the rate of a gas reaction?
How does pressure affect the rate of a gas reaction?
What is the primary reason that not all molecular collisions lead to a reaction?
What is the primary reason that not all molecular collisions lead to a reaction?
Which statement about molecular orientation in reactions is true?
Which statement about molecular orientation in reactions is true?
What effect does temperature have on molecular speed?
What effect does temperature have on molecular speed?
What is the typical energy barrier for most chemical reactions?
What is the typical energy barrier for most chemical reactions?
At what speed would a molecule with a mass of 120 amu move at 20°C according to the given data?
At what speed would a molecule with a mass of 120 amu move at 20°C according to the given data?
How does increasing temperature affect the number of reacting particles?
How does increasing temperature affect the number of reacting particles?
What does the Maxwell-Boltzmann distribution represent in reactions?
What does the Maxwell-Boltzmann distribution represent in reactions?
What is the rate law expression for the reaction where doubling [F₂] and [ClO₂] each doubles the rate?
What is the rate law expression for the reaction where doubling [F₂] and [ClO₂] each doubles the rate?
If the rate constant (k) has units of M/s, what is the reaction order if the units of k are defined as 1/(M*s)?
If the rate constant (k) has units of M/s, what is the reaction order if the units of k are defined as 1/(M*s)?
Given the rate equation k[F₂][ClO₂], what is the overall reaction order?
Given the rate equation k[F₂][ClO₂], what is the overall reaction order?
When the concentration of [A] is tripled, which correctly describes the effect on the rate if the rate law is Rate = k[A]^2?
When the concentration of [A] is tripled, which correctly describes the effect on the rate if the rate law is Rate = k[A]^2?
In the reaction F₂ + 2ClO₂ → 2ClO₂F, if doubling [ClO₂] leads to a quadrupling of the rate, what is the order with respect to [ClO₂]?
In the reaction F₂ + 2ClO₂ → 2ClO₂F, if doubling [ClO₂] leads to a quadrupling of the rate, what is the order with respect to [ClO₂]?
What effect does increasing [A] have on the reaction rate if the rate is independent of [A]?
What effect does increasing [A] have on the reaction rate if the rate is independent of [A]?
For the reaction order of BrO3- + 5Br- + 6H+ → 3 Br2 + 3H2O, if the order with respect to [BrO3-] is found to be 1, what could be a likely order for [Br-] if its increase does not affect the rate?
For the reaction order of BrO3- + 5Br- + 6H+ → 3 Br2 + 3H2O, if the order with respect to [BrO3-] is found to be 1, what could be a likely order for [Br-] if its increase does not affect the rate?
What happens to the rate if the rate constant (k) is doubled while keeping the concentrations constant?
What happens to the rate if the rate constant (k) is doubled while keeping the concentrations constant?
What does the Arrhenius equation indicate about the relationship between temperature and the kinetic rate constant (k)?
What does the Arrhenius equation indicate about the relationship between temperature and the kinetic rate constant (k)?
Which component of the Arrhenius equation represents the energy required for a reaction to occur?
Which component of the Arrhenius equation represents the energy required for a reaction to occur?
What effect do catalysts have on the activation energy of a reaction?
What effect do catalysts have on the activation energy of a reaction?
Which of the following statements is true regarding the role of catalysts in chemical reactions?
Which of the following statements is true regarding the role of catalysts in chemical reactions?
What is the molecularity of a reaction step involving three reactant molecules?
What is the molecularity of a reaction step involving three reactant molecules?
In the Arrhenius equation, what does the exponential term $e^{-Ea/RT}$ represent?
In the Arrhenius equation, what does the exponential term $e^{-Ea/RT}$ represent?
If the activation energy (Ea) is 50,000 J/mol, how is the rate of reaction affected by an increase in temperature from 293 K to 303 K?
If the activation energy (Ea) is 50,000 J/mol, how is the rate of reaction affected by an increase in temperature from 293 K to 303 K?
Which statement best describes enzymes?
Which statement best describes enzymes?
What happens to the rate of a reaction as the concentration of reactants decreases over time?
What happens to the rate of a reaction as the concentration of reactants decreases over time?
Why is molecularity not the same as reaction order?
Why is molecularity not the same as reaction order?
Flashcards
Reaction Rate
Reaction Rate
The speed at which reactants are converted into products.
Rate Law
Rate Law
A chemical reaction's rate is dependent on the concentration of reactants raised to a specific power (the order).
Order of a Reaction
Order of a Reaction
The sum of the exponents in the rate law. It tells you how much the rate increases with a change in concentration.
Catalyst
Catalyst
Signup and view all the flashcards
Activation Energy
Activation Energy
Signup and view all the flashcards
Rate Determining Step
Rate Determining Step
Signup and view all the flashcards
Rate Law for Multi-Step Reactions
Rate Law for Multi-Step Reactions
Signup and view all the flashcards
Bimolecular Step
Bimolecular Step
Signup and view all the flashcards
Reaction Mechanism
Reaction Mechanism
Signup and view all the flashcards
Reaction Order
Reaction Order
Signup and view all the flashcards
Rate Constant (k)
Rate Constant (k)
Signup and view all the flashcards
Overall Reaction Order
Overall Reaction Order
Signup and view all the flashcards
Method of Initial Rates
Method of Initial Rates
Signup and view all the flashcards
First Order Reaction
First Order Reaction
Signup and view all the flashcards
Second Order Reaction
Second Order Reaction
Signup and view all the flashcards
Zero Order Reaction
Zero Order Reaction
Signup and view all the flashcards
How does pressure affect reaction rate?
How does pressure affect reaction rate?
Signup and view all the flashcards
Surface area and reaction rates
Surface area and reaction rates
Signup and view all the flashcards
Molecular orientation and reaction rates
Molecular orientation and reaction rates
Signup and view all the flashcards
Temperature and reaction rate
Temperature and reaction rate
Signup and view all the flashcards
Transition state
Transition state
Signup and view all the flashcards
Maxwell-Boltzmann distribution
Maxwell-Boltzmann distribution
Signup and view all the flashcards
How does temperature affect the Maxwell-Boltzmann distribution?
How does temperature affect the Maxwell-Boltzmann distribution?
Signup and view all the flashcards
Determining Order of Reaction
Determining Order of Reaction
Signup and view all the flashcards
Overall Order of Reaction
Overall Order of Reaction
Signup and view all the flashcards
SN1 Reaction
SN1 Reaction
Signup and view all the flashcards
SN2 Reaction
SN2 Reaction
Signup and view all the flashcards
Elementary Reaction Rate Law
Elementary Reaction Rate Law
Signup and view all the flashcards
Rate of Reaction
Rate of Reaction
Signup and view all the flashcards
Arrhenius Equation
Arrhenius Equation
Signup and view all the flashcards
Frequency Factor (A)
Frequency Factor (A)
Signup and view all the flashcards
Enzyme
Enzyme
Signup and view all the flashcards
Molecularity
Molecularity
Signup and view all the flashcards
Termolecular Reaction
Termolecular Reaction
Signup and view all the flashcards
Elementary Reaction
Elementary Reaction
Signup and view all the flashcards
Study Notes
PHA 111: Chemical Kinetics
- This course covers chemical kinetics, focusing on reaction rates and reaction orders.
- Dr Stephen Childs, Senior Lecturer in Pharmaceutical Chemistry, is the instructor.
- Recommended reading includes Atkins' Physical Chemistry, Chapters 21.2–21.5 (9th Edition).
Why Study Reaction Rates?
- Understanding reaction mechanisms (e.g., SN1 and SN2 reactions).
- Optimizing reaction rates to improve yields and reduce side products.
- Minimizing drug degradation to predict and enhance shelf life (e.g., pH-dependent hydrolysis of aspirin).
- Understanding the driving force of reactions (e.g., H2 + ½O2 → H2O, ΔH = -286 kJ/mol).
What Affects Reaction Rate?
- Physical State: Many pharmaceutical reactions occur in solution.
- Concentration: Molecules must collide to react; increased concentration = increased collision frequency.
- Temperature: Increased frequency of collisions, increased vibrational energy of bonds.
- Catalysts: Provide alternative, faster reaction mechanisms.
Collision Theory
- Reaction rate depends on the frequency and energy of collisions between reacting molecules.
- Collision Rate Depends On:
- Concentration (higher concentration, higher collision chances).
- Pressure (for gases, higher pressure, higher rate).
- Surface Area (greater surface area, faster reaction).
- Molecular Orientation (correct orientation for successful collisions).
- Temperature (increased temperature, increased molecular speeds).
Effect of Surface Area
- Collision rate depends on the available surface area.
- Example: Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g).
Effect of Molecular Orientation
- Not all collisions result in reactions; correct molecular orientation is critical.
- Consider reactions like electrophilic addition of HCl to ethene.
- Complex reactions may need extremely specific orientations.
Effect of Molecular Speed
- Collision rate is affected by molecular speed, which is influenced by temperature.
- Temperature increases molecular speed.
- Formula for root mean square speed: Vrms = √(3RT/M).
- At 5°C Vrms = 240 m/s, at 20°C Vrms = 247 m/s (120amu).
Activation Energy
- Energy barrier associated with the transition state.
- Molecular shape distortion before the reaction.
- Typical activation energy ranges from 50 to 100 kJ.
- Average energy at 20°C is approximately 4 kJ.
- Only a small fraction of molecules have enough energy to overcome the activation energy barrier.
Maxwell-Boltzmann Distribution
- For gases, the distribution of molecular energies can be represented by a curve.
- The fraction of molecules with sufficient energy to react is proportional to the area under the curve.
Effect of Temperature
- Increasing temperature drastically increases the number of particles with sufficient activation energy.
Arrhenius Equation
- Relates the rate constant (k) to temperature (T) and activation energy (Ea).
- k = Ae-Ea/RT; A = frequency factor, Ea= activation energy, R = gas constant and T = temperature (in Kelvin).
Effect of Temperature (calculation demonstration)
- Doubling of temperature almost doubles the reaction for a fixed activation energy.
Catalysts
- Increase reaction rate without being consumed.
- Provide an alternative pathway with lower activation energy. e.g. Acid catalyzed ester hydrolysis, Chlorine radical catalyzing ozone breakdown.
Catalysts (continued)
- Do not affect the position of equilibrium; overall Gibbs free energy change (ΔG) remains unchanged.
- Enzymes are biological catalysts (proteins).
Rate of Reaction
- Measured by following concentration as a function of time.
- Decrease in reactant concentration.
- Increase in product concentration.
- Measured as change over infinitesimally small time.
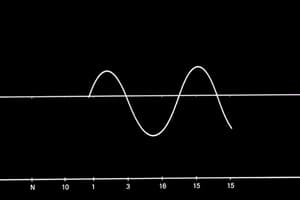
Measuring Rate of Reaction (graph)
- A graph showing reactant concentration vs time (a declining curve).
Chemical Reactions
- Multi-step processes.
- Example: NO2 reactions.
- Molecularity (number of molecules in an elementary step) – not the same as reaction order.
Rate of Reaction (effects on concentration [A])
- If rate is independent of reactant concentration [A]- nothing changes.
- If rate is dependent (proportionally) to reactant concentration [A]: Increasing [A] doubles the rate: if rate =k[A] -> doubling [A] doubles the rate Increasing [A] quadruples the rate: if rate=k[A]^2 ->doubling [A] quadruples the rate
Reaction Order (i)
- Rate law determination.
- Units of rate constant.
Reaction Order (ii)
- Calculating reaction order from experimental data.
- Example: F2 + 2ClO2 → 2ClO2F , 2nd order.
Reaction Order Example
- Calculating reaction order.
- Example: BrO3- + 5Br- + 6H+ → 3Br2 + 3H2O, orders of reactants and overall order.
Rate Law Examples
- Different scenarios and resultant order of reaction.
Reaction Mechanisms
- SN1 reaction (tert-butyl chloride and hydroxyl anion):
- Rate is dependent on concentration of substrate and not on hydroxide concentration.
- Unimolecular reaction.
- SN2 reaction (chloromethane and hydroxyl anion):
- Bimolecular reaction. Rate is dependent on concentration of substrate and hydroxide concentration
Determining Mechanisms
- Identifying the correct reaction mechanism from order of reactants.
- If the slow step comes first- reaction rates will reveal the reactants
Determining Mechanisms (continued)
- What if the slow step is not the first one?
- Need to analyze equilibrium to formulate new rate equation.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.