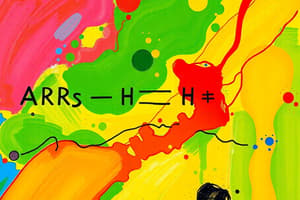
Podcast
Questions and Answers
How does increasing the temperature affect the reaction rate?
How does increasing the temperature affect the reaction rate?
- Results in fewer particles being available for collision
- Lowers the activation energy required
- Decreases the number of collisions
- Increases the kinetic energy of particles (correct)
How does decreasing particle size impact the reaction rate?
How does decreasing particle size impact the reaction rate?
- Increases surface area for more collisions (correct)
- Reduces the number of collisions
- Decreases kinetic energy of particles
- Lowers the activation energy
What happens to lipids at low temperatures according to the text?
What happens to lipids at low temperatures according to the text?
- They evaporate
- They become liquid
- They coagulate (correct)
- They solidify
How does an increase in concentration impact the rate of reaction?
How does an increase in concentration impact the rate of reaction?
What effect does an increase in surface area have on collision theory?
What effect does an increase in surface area have on collision theory?
Which of the following factors affects the rate of a chemical reaction?
Which of the following factors affects the rate of a chemical reaction?
What is the role of concentration in the collision theory of chemical reactions?
What is the role of concentration in the collision theory of chemical reactions?
According to the collision theory, for a chemical reaction to occur, what must happen to the particles involved?
According to the collision theory, for a chemical reaction to occur, what must happen to the particles involved?
In the experiments described, which setup had the fastest reaction?
In the experiments described, which setup had the fastest reaction?
What is the definition of temperature according to the text?
What is the definition of temperature according to the text?
What is the definition of concentration according to the text?
What is the definition of concentration according to the text?
Flashcards are hidden until you start studying