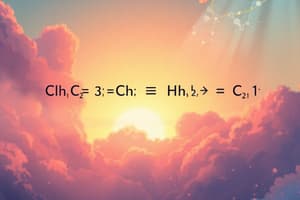Podcast
Questions and Answers
Which type of chemical reaction involves breaking complex compounds into simpler substances?
Which type of chemical reaction involves breaking complex compounds into simpler substances?
- Synthesis
- Single Replacement
- Decomposition (correct)
- Double Displacement
What is the main purpose of balancing chemical equations?
What is the main purpose of balancing chemical equations?
- To increase the reaction rate
- To represent the reaction accurately based on the law of conservation of mass (correct)
- To decrease the temperature of the reaction
- To change the stoichiometry of the reaction
In a combustion reaction, what is typically accompanied by the oxidation of a substance?
In a combustion reaction, what is typically accompanied by the oxidation of a substance?
- Absorption of energy
- Decrease in temperature
- Release of heat and often light
- Formation of water (correct)
Which aspect of chemistry allows us to determine the amounts of reactants and products in a chemical reaction?
Which aspect of chemistry allows us to determine the amounts of reactants and products in a chemical reaction?
What type of reaction involves swapping atoms between two compounds to form two new compounds?
What type of reaction involves swapping atoms between two compounds to form two new compounds?
In a neutralization reaction, what is formed when an acid reacts with a base?
In a neutralization reaction, what is formed when an acid reacts with a base?
What does a catalyst do in a chemical reaction?
What does a catalyst do in a chemical reaction?
In a chemical equation, what does stoichiometry help us calculate?
In a chemical equation, what does stoichiometry help us calculate?
What is chemical equilibrium characterized by?
What is chemical equilibrium characterized by?
How does a catalyst affect the activation energy of a reaction?
How does a catalyst affect the activation energy of a reaction?
What can shift the equilibrium position of a reaction?
What can shift the equilibrium position of a reaction?
What is the role of chemical equations in visualizing reactions?
What is the role of chemical equations in visualizing reactions?
Flashcards are hidden until you start studying
Study Notes
Types of Chemical Reactions
Chemical reactions can be classified into various types based on the reactants and their behavior during the process. Understanding these types is crucial for predicting reaction outcomes and balancing chemical equations.
- Synthesis: Forming new chemical compounds from simpler substances, also known as composition.
- Decomposition: Breaking complex compounds into simpler substances.
- Single Replacement: Replacing one element in a compound with another element, forming two new compounds.
- Double Displacement (Metathesis): Swapping atoms between two compounds to form two new compounds.
- Combustion: Oxidation of a substance, typically accompanied by the release of heat and often light.
- Neutralization: The reaction between an acid and a base to form a salt and water.
Balancing Chemical Equations
Balancing chemical equations is essential for representing the reaction accurately, as dictated by the law of conservation of mass. The balanced chemical equation will have the same number of atoms of each element on both sides of the equation, and the sum of the atomic masses will be equal on both sides.
For example, the balanced chemical equation for the combustion of glucose is:
[ \ce{C_6H_{12}O6(s) + 6O2(g) \rightarrow 6CO2(g) + 6H2O(l)} ]
Chemical Equations and Stoichiometry
Stoichiometry, the quantitative aspect of chemistry, allows us to determine the amounts of reactants and products in a chemical reaction. Chemical equations help us visualize the mole ratio of reactants to products. For example, in the glucose combustion reaction above, the mole ratio of glucose to oxygen is 1:6.
Stoichiometry also helps us calculate the mass of reactants and products required or produced in a reaction, based on their molecular weights.
Catalysts in Chemical Reactions
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They do this by providing an alternative reaction pathway with a lower activation energy. The catalyst facilitates the reaction but does not affect the equilibrium position or the products formed.
Chemical Equilibrium
Chemical equilibrium is a state in which the forward and reverse reactions reach an equilibrium constant, and the rate of the forward reaction equals the rate of the reverse reaction. At equilibrium, the concentrations of reactants and products do not change.
The expression for equilibrium constant, K, is:
[ K = \frac{[\text{products}]^n}{[\text{reactants}]^m} ]
where n and m are the stoichiometric coefficients in the balanced chemical equation.
Equilibrium can be shifted by changing the concentration of reactants or products, temperature, or pressure. Reversible reactions can return to equilibrium after the shift, reaching a new equilibrium position.
Understanding these key concepts of chemical reactions and equations will enable you to analyze and predict the behavior of chemical systems, from the preparation of everyday chemicals to the operation of industrial reactors.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.