Podcast
Questions and Answers
What is the main purpose of rate laws in chemistry?
What is the main purpose of rate laws in chemistry?
- To determine the concentration of products at a specific time
- To measure the initial concentration of reactants
- To predict the exact time at which a reaction will proceed
- To calculate reaction rates and identify reaction orders (correct)
How can the order of a reaction be recognized using rate laws?
How can the order of a reaction be recognized using rate laws?
- From a plot of either rate vs. concentration or concentration vs. time (correct)
- By measuring the initial rate of the reaction
- From a plot of concentration vs. time at a particular time
- By calculating the half-life of the reaction
What does the initial rate of a reaction measure?
What does the initial rate of a reaction measure?
- The rate of reaction at very short times before significant concentration changes occur (correct)
- The rate of reaction at the end of the reaction
- The maximum rate of the reaction
- The average rate of the reaction throughout its progress
How are integrated rate laws used in solving for the half-life of a reaction?
How are integrated rate laws used in solving for the half-life of a reaction?
Why is it important to understand the difference between differential and integrated rate laws?
Why is it important to understand the difference between differential and integrated rate laws?
What is the purpose of rate laws in the study of chemical kinetics?
What is the purpose of rate laws in the study of chemical kinetics?
What does the rate constant 'k' represent in a rate law?
What does the rate constant 'k' represent in a rate law?
How is the value of 'n' related to the reaction stoichiometry in a rate law?
How is the value of 'n' related to the reaction stoichiometry in a rate law?
How is the rate constant 'k' typically determined?
How is the rate constant 'k' typically determined?
In what units is the rate constant 'k' expressed for a first-order reaction?
In what units is the rate constant 'k' expressed for a first-order reaction?
Flashcards are hidden until you start studying
Study Notes
In the study of chemical kinetics, rate laws are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentrations of the reactants and products in the reaction. These laws provide a quantitatively description of how changes in the amount of a substance affect the rate of a chemical reaction and are used to calculate reaction rates and identify reaction orders and derive rate laws from rate and concentration data.
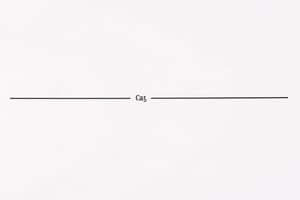
A rate law generally has the form:
rate = k[A]ⁿ
- k: rate constant, a proportionality constant in the relationship between reaction rate and concentrations of reactants
- [A]ⁿ: concentration of reactant A to the power of n, where n is the reaction order
The value of n is not related to the reaction stoichiometry and must be determined by experiment to understand the dependence of the reaction rate on the concentrations of the species and the order of the reaction that leads to various differential rate laws and integrated rate laws for different scenarios.
The rate constant k is usually determined by experiment and can be expressed in different units depending on the reaction order and the units of time used (e.x. mol/L/s for zero order, s−1 for first order, L/mol/s for second order, and mol−2 L2 s−1 for the case of a reaction with three reactants and molarity (M) instead of mol/L for the units of k).
Rate laws are used to calculate reaction rates and identify reaction orders by:
- Understand the definition of the reaction rate and be able to write down a differential equation
- Understand the difference between the differential vs. integrated rate laws and be able to recognize the order of a reaction from a plot of either rate vs. concentration or concentration vs. time
- Be able to use the integrated rate laws to solve for the half- life
- Be able to solve the integrated rate laws algebraically, given the initial concentration, to find the concentration at a later time
The initial rate of a reaction can be determined by measuring the rate of reaction at very short times before any significant changes in concentration occur and can be expressed as the derivative of concentration with respect to time or the slope of a graph of concentration against time at a particular time. As a reaction progresses, the concentrations of both reactants and products change, and the rate of the reaction depends on the concentrations of the reactants and the order of the reaction [100] [101] [102] [103] [104] [105] [106] [107] [108] [109] [110] [111] [112] [113] [114] [115] [116] [117] [118] [119] [120] [121] [122] [123] [124] [125] [126] [127] [128] [129] [130] [131] [132] [133] [134] [135] [136] [137] [138] [139] [140] [141] [142] [143] [144] [145] [146] [147] [148] [149] [150] [151] [152] [153] [154] [155] [156] [157] [158] [159] [160] [161] [162] [163] [164] [165] [166] [167] [168] [169] [170] [171] [172] [173] [174] [175] [176] [177] [178] [179] [180] [181] [182] [183] [184] [185] [186] [187] [188] [189] [190] [191] [192] [193] [194] [195] [196] [197] [198] [199] [200] [201] [202] [203] [204] [205] [206] [207] [208] [209] [210] [211] [212] [213] [214] [215] [216] [217] [218] [219] [220] [221] [222] [223] [224] [225] [226] [227] [228] [229] [230] [231] [232] [233] [234] [235] [236] [237] [238] [239] [240] [241] [242] [243] [244] [245] [246] [247] [248] [249] [250] [251] [252] [253] [254] [255] [256] [257] [258] [259] [260] [261] [262] [263] [264] [265] [266] [267] [268] [269] [270] [271] [272] [273] [274] [275] [276] [277] [278] [279] [280] [281] [282] [283] [284] [285] [286] [287] [288] [289] [290] [291] [292] [293] [294] [295] [296] [297] [298] [299] [300] [301] [302
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.