Podcast
Questions and Answers
What is the molar flow rate of CO in the fresh feed?
What is the molar flow rate of CO in the fresh feed?
186 mol/min
What is the molar flow rate of H2 in the fresh feed?
What is the molar flow rate of H2 in the fresh feed?
30 mol/min
What is the production rate of methanol?
What is the production rate of methanol?
1.02 mol/min
Flashcards are hidden until you start studying
Study Notes
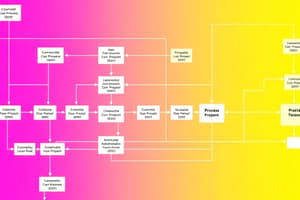
Methanol Production Process
- Methanol is produced by reacting carbon monoxide with hydrogen.
Reactor Outlet Stream
- The outlet stream of the reactor flows at a rate of 300 mol/min.
- The stream composition is:
- 10% by weight of H2
- 62.0% by weight of CO
- 28% by weight of CH3OH
Recycle Stream
- The fraction of methanol in the recycle stream is 0.006.
Process Characteristics
- CO and H2 are not consumed in the process.
- CH3OH is not consumed in the process.
- A portion of the methanol leaving the reactor condenses and is recycled back to the reactor.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.