Podcast
Questions and Answers
What are amines derivatives of?
What are amines derivatives of?
- Hydrochloric acid (HCl)
- Ammonia (NH3) (correct)
- Water (H2O)
- Ethanol (C2H5OH)
What is the IUPAC name for CH3-CH2-CH2-CH2-N-CH3?
What is the IUPAC name for CH3-CH2-CH2-CH2-N-CH3?
N-methyl-1-butanamine
What is the common name for CH3-CH2-NH2?
What is the common name for CH3-CH2-NH2?
- Butylamine
- Dimethylamine
- Ethylamine (correct)
- Propylamine
What is a primary amine?
What is a primary amine?
What is a secondary amine?
What is a secondary amine?
What is a tertiary amine?
What is a tertiary amine?
What is the amine of benzene called?
What is the amine of benzene called?
Which compound is more soluble in water, 1° amines or 3° amines?
Which compound is more soluble in water, 1° amines or 3° amines?
Amines react as Brønsted-Lowry acids in water.
Amines react as Brønsted-Lowry acids in water.
When does an amine salt form?
When does an amine salt form?
What are alkaloids?
What are alkaloids?
Amines are soluble in water if they have __ to __ carbon atoms.
Amines are soluble in water if they have __ to __ carbon atoms.
What happens when ethylamine reacts with H2O?
What happens when ethylamine reacts with H2O?
Which of the following is a characteristic of amine salts?
Which of the following is a characteristic of amine salts?
Flashcards are hidden until you start studying
Study Notes
Amines Overview
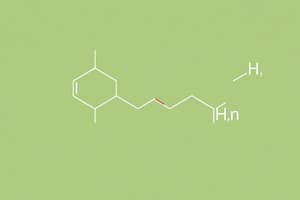
- Amines are derivatives of ammonia (NH3) containing one or more alkyl or aryl groups.
- The IUPAC naming convention replaces the "e" in the alkane name with "amine".
- Primary amines feature one carbon group bonded to nitrogen, secondary amines have two, and tertiary amines have three.
IUPAC Naming of Amines
- Identify the longest carbon chain connected to the nitrogen atom.
- Replace the "e" of the alkane name with "amine" and number the chain for the position of the amine group.
- Alkyl groups attached to nitrogen are prefixed with "N-" followed by the alkyl name.
Examples of Naming and Classifying Amines
- N-methyl-1-butanamine is derived from a butane structure with a methyl group on nitrogen.
- Common names for amines include Ethylamine (CH3-CH2-NH2) and Dimethylamine (CH3-NH-CH3).
- Ethylmethylamine (common) and N-methylethanamine (IUPAC) are also examples.
Structural Representation
- Condensed structural formulas showcase the arrangement of amine groups within a carbon chain.
Functional Groups and Priority in Naming
- Compounds containing multiple functional groups prioritize oxygen-containing groups over amine groups.
Special Cases
- Aniline is the amine derived from benzene.
- Amines can become amine salts when neutralized by an acid, often leading to names that include "ammonium" followed by a negative ion.
Solubility and Properties of Amines
- Amines with 1 to 6 carbon atoms are generally water-soluble, with primary amines being most soluble.
- Weak hydrogen bonding in amines results from nitrogen being less electronegative than oxygen.
Reactions and Characteristics
- Amines act as Brønsted-Lowry bases in water and equipotently attract protons from water.
- Properties of amine salts include being ionic compounds, soluble in water, and often used as pharmaceuticals.
Heterocyclic Amines
- Heterocyclic amines contain nitrogen in a cyclic structure with five or six atoms and play significant biological roles, such as in DNA and RNA.
Alkaloids and Their Application
- Alkaloids are bioactive nitrogen-containing compounds produced by plants.
- These compounds have various uses, including stimulants and pain relief (e.g., nicotine, caffeine, morphine).
Pharmacology and Drug Design
- Research in pharmacology focuses on designing and modifying drugs that retain beneficial properties of alkaloids while minimizing addictive effects.
Amides
- Amides are formed when an amino group (-NH2) replaces the -OH group in carboxylic acids, creating a distinct class of compounds.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.