Podcast
Questions and Answers
What prefix is used to denote the D series of carbohydrates?
What prefix is used to denote the D series of carbohydrates?
- S-
- R-
- L-
- D- (correct)
Which term describes the optical activity of a compound that rotates plane polarized light to the left?
Which term describes the optical activity of a compound that rotates plane polarized light to the left?
- Diastereomeric
- Enantiomeric
- Dextrorotatory
- Levorotatory (correct)
What is a key characteristic of diastereomers?
What is a key characteristic of diastereomers?
- They are mirror images of each other.
- They differ in one or more chiral centers. (correct)
- They must have the same chemical and physical properties.
- They are always optically active.
What describes a special subclass of diastereomers that differ at only one chiral center?
What describes a special subclass of diastereomers that differ at only one chiral center?
In sugar cyclization, which type of structure is formed by 5 and 6 carbon sugars?
In sugar cyclization, which type of structure is formed by 5 and 6 carbon sugars?
What classification is given to an optically active compound with the ability to rotate polarized light?
What classification is given to an optically active compound with the ability to rotate polarized light?
How does a Dextrorotatory compound rotate polarized light?
How does a Dextrorotatory compound rotate polarized light?
What does the Haworth projection help with regarding carbohydrates?
What does the Haworth projection help with regarding carbohydrates?
What type of polysaccharide is composed of only one kind of monosaccharide unit?
What type of polysaccharide is composed of only one kind of monosaccharide unit?
Which projection represents the cyclic form of sugar molecules?
Which projection represents the cyclic form of sugar molecules?
What is the primary property of a chiral carbon atom?
What is the primary property of a chiral carbon atom?
What are enantiomers characterized by?
What are enantiomers characterized by?
How do you determine the number of stereoisomers for a compound?
How do you determine the number of stereoisomers for a compound?
What type of isomers possess the same molecular formula but have different functional groups?
What type of isomers possess the same molecular formula but have different functional groups?
What is used to denote enantiomers in carbohydrate chemistry?
What is used to denote enantiomers in carbohydrate chemistry?
What do heteropolysaccharides consist of?
What do heteropolysaccharides consist of?
What determines the optical activity of the α- and β-anomers?
What determines the optical activity of the α- and β-anomers?
Which of the following correctly describes mutarotation?
Which of the following correctly describes mutarotation?
What is the significance of the anomeric carbon in sugar chemistry?
What is the significance of the anomeric carbon in sugar chemistry?
Which of the following statements is true regarding the solubility of monosaccharides in water?
Which of the following statements is true regarding the solubility of monosaccharides in water?
Which pair of isomers are classified as enantiomers?
Which pair of isomers are classified as enantiomers?
What is one effect of having different enantiomers in biological systems?
What is one effect of having different enantiomers in biological systems?
Which process is specifically mentioned as a reaction of monosaccharides?
Which process is specifically mentioned as a reaction of monosaccharides?
What happens to the specific optical rotation of a solution of α- and β-glucopyranose over time?
What happens to the specific optical rotation of a solution of α- and β-glucopyranose over time?
What is the product of reducing the carbonyl group in a monosaccharide?
What is the product of reducing the carbonyl group in a monosaccharide?
Which type of acid is formed from oxidation of reducing sugars?
Which type of acid is formed from oxidation of reducing sugars?
What structural change occurs in a carbohydrate when it forms an amino sugar?
What structural change occurs in a carbohydrate when it forms an amino sugar?
What occurs during the process of mutarotation in carbohydrates?
What occurs during the process of mutarotation in carbohydrates?
What describes a glycoside?
What describes a glycoside?
What is the main function of aldose-ketose isomerization?
What is the main function of aldose-ketose isomerization?
Which of the following statements regarding sugar alcohols is NOT correct?
Which of the following statements regarding sugar alcohols is NOT correct?
What is a common reagent used for the identification of reducing sugars?
What is a common reagent used for the identification of reducing sugars?
What is the primary function of carbohydrates in plants and animals?
What is the primary function of carbohydrates in plants and animals?
What is the simplest form of monosaccharides?
What is the simplest form of monosaccharides?
Which of the following best describes oligosaccharides?
Which of the following best describes oligosaccharides?
Which classification of carbohydrates is characterized by having more than 10 monosaccharide units?
Which classification of carbohydrates is characterized by having more than 10 monosaccharide units?
What suffix is generally used to indicate a carbohydrate molecule?
What suffix is generally used to indicate a carbohydrate molecule?
What distinguishes monosaccharides from other types of carbohydrates?
What distinguishes monosaccharides from other types of carbohydrates?
Which example of carbohydrate serves as a structural component in plants?
Which example of carbohydrate serves as a structural component in plants?
What is the molecular formula representation of carbohydrates, assuming the hydrolysis behavior is considered?
What is the molecular formula representation of carbohydrates, assuming the hydrolysis behavior is considered?
Study Notes
Carbohydrates: Introduction
- Most abundant naturally occurring organic compounds.
- Produced via photosynthesis.
- Historically named "carbohydrates" due to the H₂O:C ratio resembling water.
- Modern definition expands beyond Cn(H₂O)n.
- Serve crucial roles in plants and animals (energy storage, structural support).
- Essential for various industries: sugar, starch, paper, textiles, food processing, pharmaceuticals.
- Found in nucleic acids (2-deoxy-D-ribose).
- Some medicines are carbohydrate-based.
Carbohydrate Classification
- Classified by hydrolysis behavior:
- Monosaccharides: Simplest sugars, not further hydrolyzable.
- Oligosaccharides: 2-10 monosaccharide units, mostly hexoses.
- Polysaccharides: More than 10 monosaccharide units.
Monosaccharides
- Crystalline, water-soluble, sweet-tasting.
- Polyhydroxy aldehydes or polyhydroxy ketones with 3+ carbons.
- Classified by:
- Number of carbons.
- Presence of aldehyde or keto group.
- Suffix "-ose" denotes a carbohydrate.
- Simplest examples: glyceraldehyde and dihydroxyacetone (trioses).
Oligosaccharides
- Glycosidic linkages of 2-10 monosaccharide units.
- Sub-classified by number of units: disaccharides, trisaccharides, tetrasaccharides (e.g., stachyose).
Polysaccharides
- Non-sweet, high molecular weight.
- Long chains (hundreds/thousands of monosaccharide units), linear or branched.
- Two types:
- Homopolysaccharides (homoglycans): One type of monosaccharide.
- Heteropolysaccharides (heteroglycans): Two or more types of monomers.
- Derived carbohydrates yield monosaccharides + derivatives (sulfate esters, amino-sugars, uronic acids).
Drawing Sugar Molecules
- Three methods:
- Fischer projection: Linear structure, shows configuration.
- Haworth projection: Cyclic forms, shows relative stereochemistry.
- Chair/boat conformational: Represents 3D shapes.
Fischer Projection
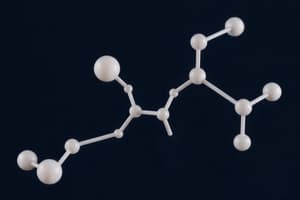
- Ball-and-stick models show actual molecular configuration.
- Shows solid and dashed wedges for bond direction.
- Highlights chiral carbon atoms (four different attached groups).
Isomerism
- Isomers: Same molecular formula, different structures/orientation.
- Structural (constitutional) isomers: Different structures/functional groups.
- Stereoisomers: Same molecular and structural formula, different spatial arrangements. Number of stereoisomers = 2ⁿ (n = number of chiral centers).
- Enantiomers (optical isomers): Mirror images, non-superimposable (D and L isomers). Number of enantiomeric pairs = 2ⁿ/2.
- Diastereomers: Differ in one or more chiral centers, not mirror images. Have different physical/chemical properties.
- Epimers: Diastereomers differing in stereochemistry at one chiral center.
Enantiomers
- Mirror images, non-superimposable.
- Designated D and L isomers.
- Same chemical/physical properties except optical rotation of plane-polarized light.
- D/L assignment based on the highest-numbered chiral center (configurational atom): OH to the right (D), OH to the left (L).
Optical Activity
- Optically active compounds rotate plane-polarized light (clockwise = dextrorotatory (+), anticlockwise = levorotatory (-)).
- Measured using a polarimeter.
- Most naturally occurring carbohydrates have D-configuration.
Diastereomers
- Differ in one or more chiral centers but are not mirror images.
- Have different physical and chemical properties and names.
Sugar Cyclization
- 5 and 6-carbon sugars spontaneously cyclize to furanose (5-membered ring) or pyranose (6-membered ring) forms.
- Involves formation of hemiacetals (aldose) or hemiketals (ketose).
- Process is spontaneous and reversible.
Haworth Projection
- Represents cyclic forms.
- Rules: OH to right in Fischer → OH down in Haworth; OH to left in Fischer → OH up in Haworth.
Anomers
- New stereoisomers created during ring closure (α- and β- anomers).
- Anomeric carbon: New chiral center formed.
Mutarotation
- Interconversion of α- and β- anomers in solution, reaching equilibrium.
- Changes optical rotation over time.
Physical Properties of Monosaccharides
- Solids at room temperature (crystalline).
- Highly water-soluble due to many OH groups.
- Mostly sweet-tasting.
- Optically active.
- Many exhibit mutarotation.
Monosaccharide Reactions
- Esterification: Formation of phosphate esters (e.g., glucose 6-phosphate).
- Glycoside formation: Linkage of monosaccharides via glycosidic bonds.
- Reduction: Carbonyl group reduction to hydroxyl (forms sugar alcohols/alditols, e.g., sorbitol, erythritol).
- Oxidation: Yields aldonic, aldaric (saccharic), or uronic acids, depending on oxidizing agent. Reducing sugars reduce mild oxidizing agents (Fehlings', Benedict's, Tollens' reagents).
- Reaction with alkalis: Aldose-ketose isomerization and aldose epimerization.
- Formation of amino derivatives: Replacement of a hydroxyl group with an amino group (e.g. forming amino sugars, often at C2).
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.
Related Documents
Description
Explore the fascinating world of carbohydrates, the most abundant organic compounds. This quiz covers their classification, from monosaccharides to polysaccharides, and highlights their importance in various industries and biological processes.