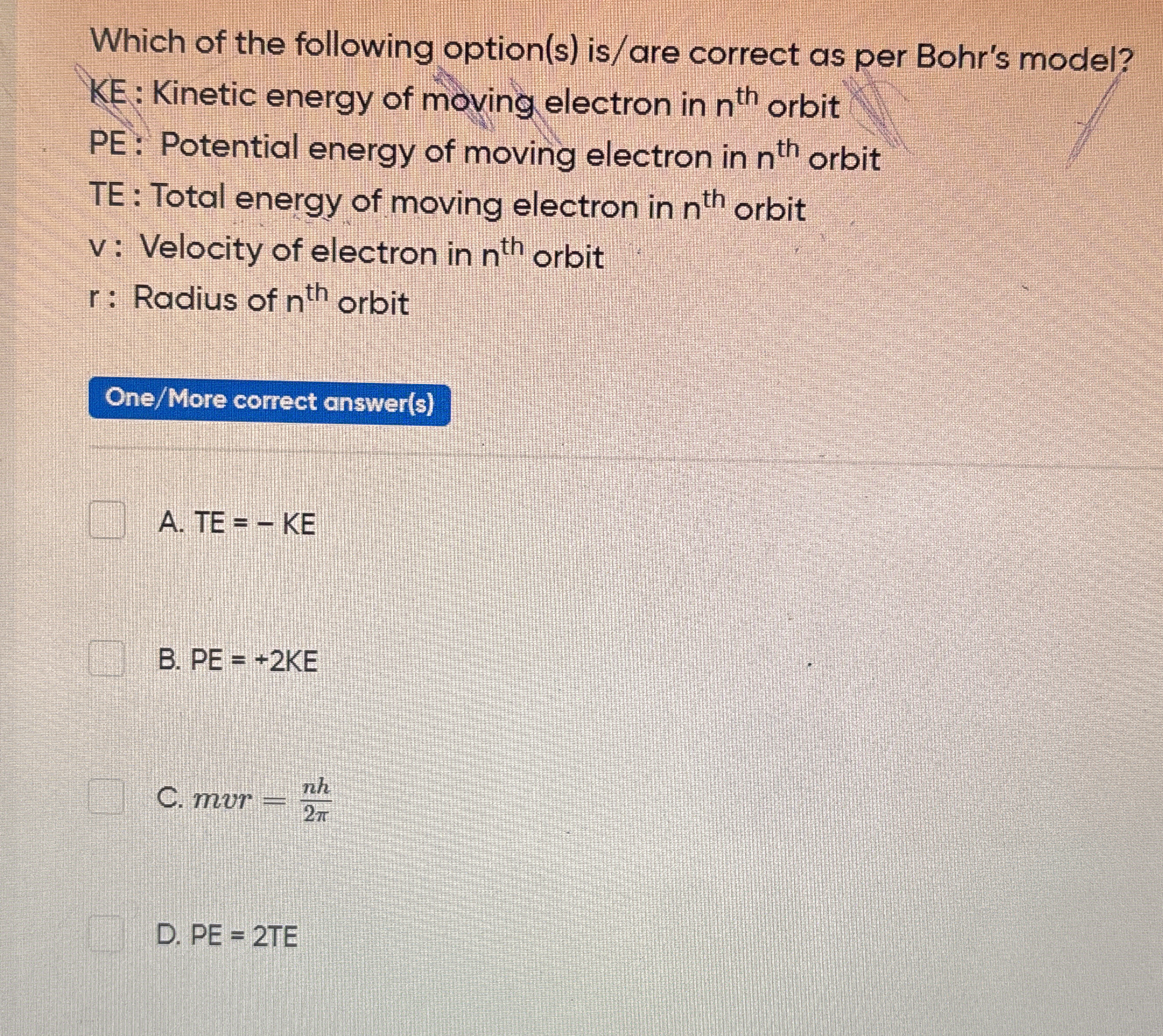
Which of the following option(s) is/are correct as per Bohr's model?
Understand the Problem
The question is asking which of the provided mathematical relationships are correct according to Bohr's model of the atom, specifically in relation to kinetic energy, potential energy, total energy, and other variables related to an electron in a specific orbit.
Answer
A: TE = - KE, B: PE = +2KE
The correct options are A: TE = - KE and B: PE = +2KE.
Answer for screen readers
The correct options are A: TE = - KE and B: PE = +2KE.
More Information
Bohr’s model provides quantized energy levels for electrons and helps describe atomic behavior accurately. It establishes relations for energy, velocity, and angular momentum in atomic orbits.
Tips
A common mistake is confusing the formulas for TE and PE. Remember that TE is the sum of KE and PE but is negative in terms of KE.
AI-generated content may contain errors. Please verify critical information