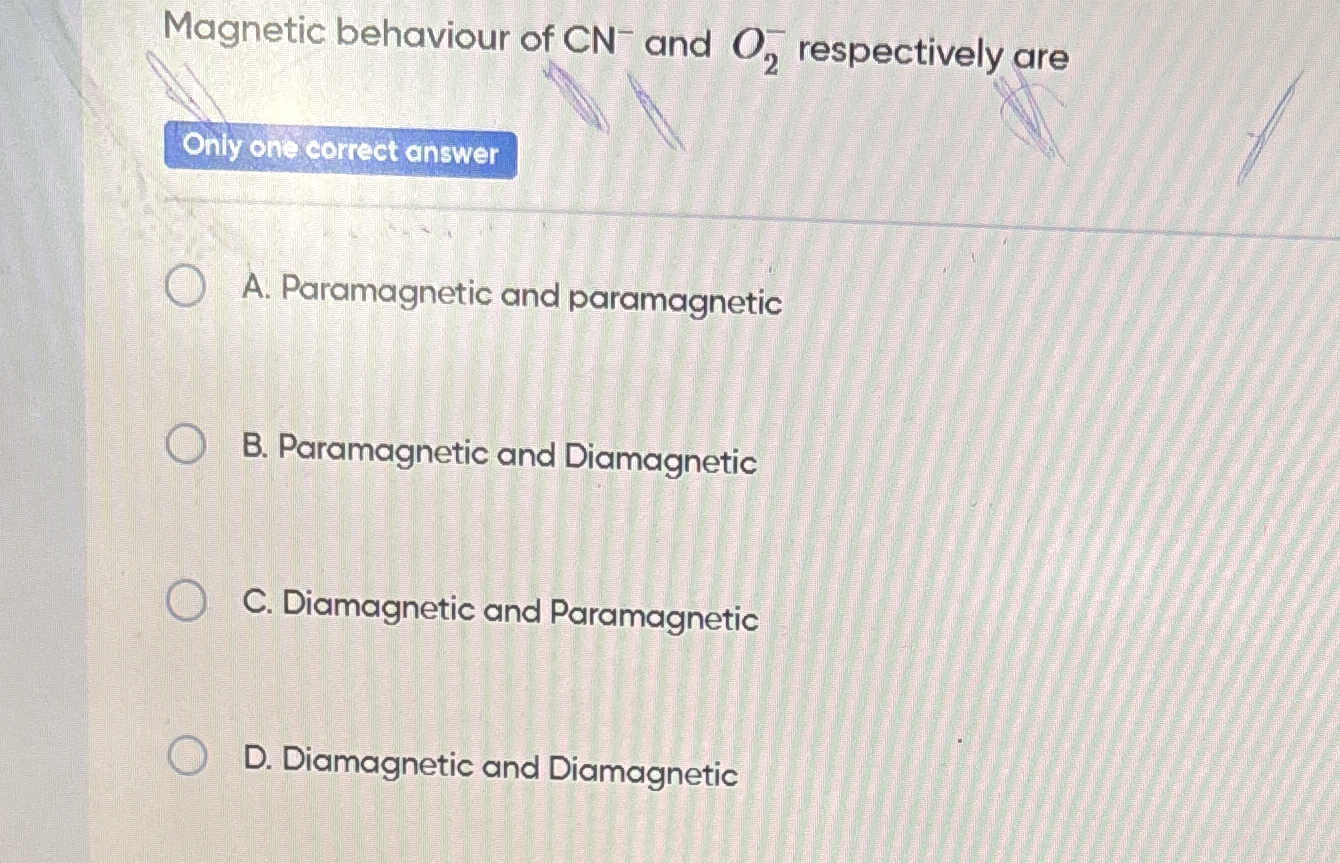
Magnetic behaviour of CN- and O2 respectively are?
Understand the Problem
The question is asking about the magnetic behavior of CN⁻ and O₂, specifically how they are classified as paramagnetic or diamagnetic. The user is required to choose one correct answer from the provided options.
Answer
C: Diamagnetic and Paramagnetic
The final answer is C: Diamagnetic and Paramagnetic.
Answer for screen readers
The final answer is C: Diamagnetic and Paramagnetic.
More Information
CN- is diamagnetic because it has paired electrons, while O2 is paramagnetic due to its unpaired electrons.
Tips
A common mistake is not considering the electronic configuration for magnetic properties.
Sources
AI-generated content may contain errors. Please verify critical information