How do the properties of water relate to its roles in living organisms?
Understand the Problem
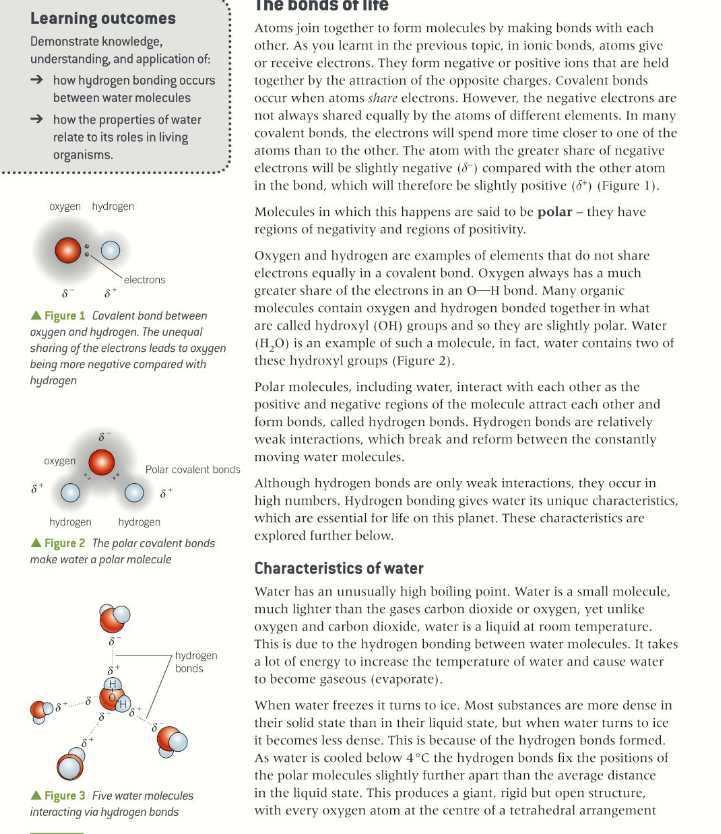
The question is outlining the properties of water molecules and how hydrogen bonding occurs, particularly in living organisms. It emphasizes the polar nature of water and its unique characteristics due to hydrogen bonds.
Answer
Water's properties support life through nutrient transport, temperature regulation, and suitable conditions for reactions.
The properties of water, such as cohesion, adhesion, thermal properties, and its role as a solvent, are essential for processes like nutrient transport, temperature regulation, and creating suitable environments for reactions in living organisms.
Answer for screen readers
The properties of water, such as cohesion, adhesion, thermal properties, and its role as a solvent, are essential for processes like nutrient transport, temperature regulation, and creating suitable environments for reactions in living organisms.
More Information
Water's cohesive and adhesive properties, due to hydrogen bonding, enable capillary action, which is crucial for nutrient transport in plants. Its high specific heat capacity stabilizes temperatures, benefiting ecosystems.
Tips
Remember that water's polarity is key to its solvent properties. Common mistakes include overlooking the role of hydrogen bonds in its thermal properties.
Sources
- Biological Roles of Water: Why is water necessary for life? - sitn.hms.harvard.edu
- Lesson summary: Water and life (article) | Khan Academy - khanacademy.org
AI-generated content may contain errors. Please verify critical information