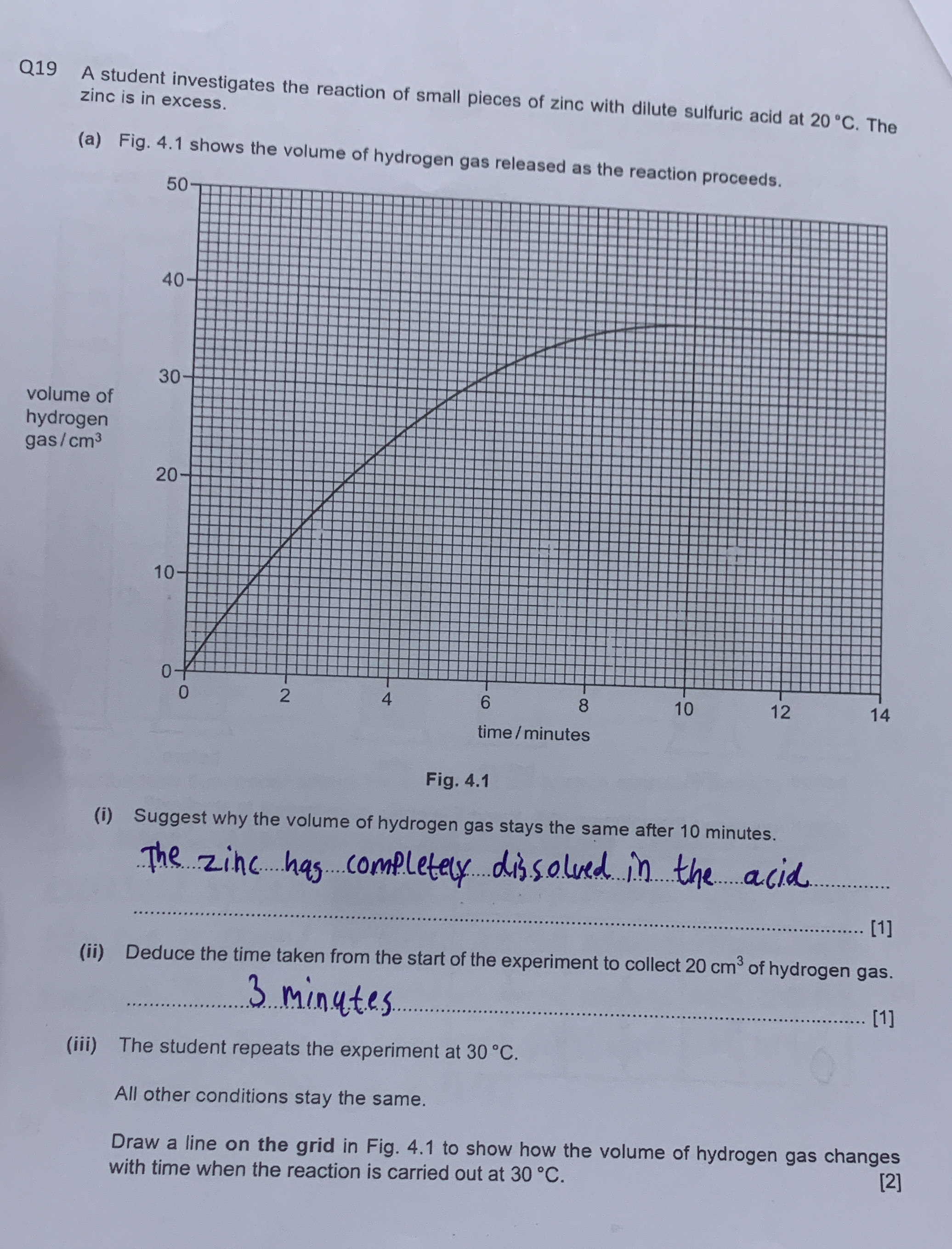
A student investigates the reaction of small pieces of zinc with dilute sulfuric acid at 20 °C. The zinc is in excess. Fig. 4.1 shows the volume of hydrogen gas released as the rea... A student investigates the reaction of small pieces of zinc with dilute sulfuric acid at 20 °C. The zinc is in excess. Fig. 4.1 shows the volume of hydrogen gas released as the reaction proceeds. (i) Suggest why the volume of hydrogen gas stays the same after 10 minutes. (ii) Deduce the time taken from the start of the experiment to collect 20 cm³ of hydrogen gas. (iii) The student repeats the experiment at 30 °C. Draw a line on the grid in Fig. 4.1 to show how the volume of hydrogen gas changes with time when the reaction is carried out at 30 °C.
Understand the Problem
The question involves the reaction between zinc and dilute sulfuric acid, specifically analyzing a graph showing hydrogen gas production over time and estimating the effects of conditions on this reaction.
Answer
(i) Acid is used up. (ii) 3 minutes. (iii) Steeper curve at 30°C.
(i) The volume stays the same because the sulfuric acid is fully consumed. (ii) The time to collect 20 cm³ is 3 minutes. (iii) At 30°C, the line will have a steeper slope initially and level off earlier due to a faster reaction rate.
Answer for screen readers
(i) The volume stays the same because the sulfuric acid is fully consumed. (ii) The time to collect 20 cm³ is 3 minutes. (iii) At 30°C, the line will have a steeper slope initially and level off earlier due to a faster reaction rate.
More Information
Typically, increasing temperature speeds up chemical reactions, producing hydrogen gas more rapidly.
Tips
Remember reaction rates increase with temperature. Ensure accuracy when reading values from graphs.
Sources
- PDF Cambridge IGCSE™ - cambridgeinternational.org
- Gauthmath solution - gauthmath.com
AI-generated content may contain errors. Please verify critical information